3,5-DimethoxybenzylalcoholCAS# 705-76-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 705-76-0 | SDF | Download SDF |
| PubChem ID | 69718 | Appearance | Cryst. |
| Formula | C9H12O3 | M.Wt | 168.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
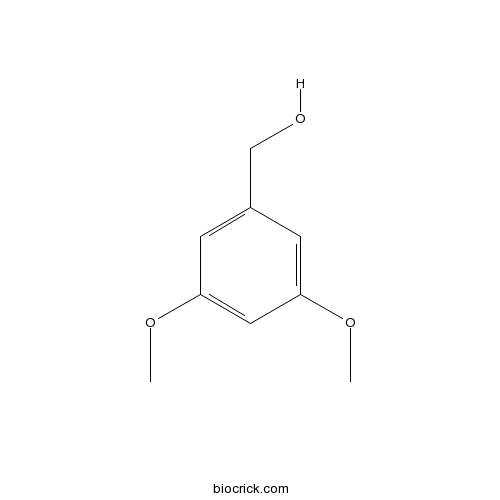
| Chemical Name | (3,5-dimethoxyphenyl)methanol | ||
| SMILES | COC1=CC(=CC(=C1)CO)OC | ||
| Standard InChIKey | AUDBREYGQOXIFT-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,5-Dimethoxybenzylalcohol is a natural product from Pinus sylvestris. |
| Kinase Assay | Experimental and Theoretical Studies on the Pyrolysis Mechanism of β-1-Type Lignin Dimer Model Compound[Reference: WebLink]BioResources,2016,11(3):6232-43.A β-1-type lignin dimer, 1,2-bis(3,5-dimethoxyphenyl)propane-1,3-diol was employed as a model compound in this study.
|

3,5-Dimethoxybenzylalcohol Dilution Calculator

3,5-Dimethoxybenzylalcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9453 mL | 29.7265 mL | 59.453 mL | 118.9061 mL | 148.6326 mL |
| 5 mM | 1.1891 mL | 5.9453 mL | 11.8906 mL | 23.7812 mL | 29.7265 mL |
| 10 mM | 0.5945 mL | 2.9727 mL | 5.9453 mL | 11.8906 mL | 14.8633 mL |
| 50 mM | 0.1189 mL | 0.5945 mL | 1.1891 mL | 2.3781 mL | 2.9727 mL |
| 100 mM | 0.0595 mL | 0.2973 mL | 0.5945 mL | 1.1891 mL | 1.4863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2'-Hydroxy-5'-methoxyacetophenone
Catalog No.:BCN4270
CAS No.:705-15-7
- ARP 100
Catalog No.:BCC2370
CAS No.:704888-90-4
- Mitoxantrone HCl
Catalog No.:BCC4924
CAS No.:70476-82-3
- Petasinoside
Catalog No.:BCN1989
CAS No.:70474-34-9
- Petasinine
Catalog No.:BCN1988
CAS No.:70474-33-8
- Schizanthine A
Catalog No.:BCN1936
CAS No.:70474-24-7
- Corymbol
Catalog No.:BCN6617
CAS No.:7047-54-3
- Norfloxacin
Catalog No.:BCC4688
CAS No.:70458-96-7
- Pefloxacin Mesylate
Catalog No.:BCC4821
CAS No.:70458-95-6
- Pefloxacin
Catalog No.:BCC4231
CAS No.:70458-92-3
- (+)-MK 801
Catalog No.:BCC1288
CAS No.:70449-94-4
- 17alpha-Neriifolin
Catalog No.:BCN4269
CAS No.:7044-31-7
- α-Conotoxin PnIA
Catalog No.:BCC5978
CAS No.:705300-84-1
- NS 3763
Catalog No.:BCC7275
CAS No.:70553-45-6
- Aflatrem
Catalog No.:BCN7414
CAS No.:70553-75-2
- Daurisoline
Catalog No.:BCN2675
CAS No.:70553-76-3
- Herbimycin A
Catalog No.:BCC7132
CAS No.:70563-58-5
- Yunaconitine
Catalog No.:BCN6261
CAS No.:70578-24-4
- Shizukanolide A
Catalog No.:BCN8021
CAS No.:70578-36-8
- Obtusin
Catalog No.:BCC8223
CAS No.:70588-05-5
- Chrysoobtusin
Catalog No.:BCC8309
CAS No.:70588-06-6
- 19alpha-Hydroxyfern-7-ene
Catalog No.:BCN7405
CAS No.:70588-12-4
- 14beta-Benzoyloxy-2-deacetylbaccatin VI
Catalog No.:BCN1373
CAS No.:705973-69-9
- Phlorizin dihydrate
Catalog No.:BCN2584
CAS No.:7061-54-3
The effects of exposure duration and feeding status on fish bile metabolites: implications for biomonitoring.[Pubmed:9515087]
Ecotoxicol Environ Saf. 1998 Feb;39(2):147-53.
Biliary metabolites of 2-chlorosyringaldehyde (2-CSA), the major chlorinated phenol found in chlorine dioxide bleached eucalypt pulp effluent, have been found to be sensitive biomarkers of effluent exposure in the sand flathead (Platycephalus bassensis). Before this method of biomonitoring can be applied in the field, the influences of exposure duration, depuration time, and fish feeding status on the level of this metabolite should be determined. In this study, sand flathead were exposed to a measured concentration of 0.3 microgram/1 of 2-CSA for 1, 2, 4, 8, 12, or 16 days. Fish previously exposed to 2-CSA were then held in sea-water alone for 1, 2, 3, 4, or 6 days. Fish were fed ad libitum throughout the experiment, and the fullness of the fish's stomach at the time of sampling was noted. There were no effects of exposure on biotransformation enzyme activities, either between exposure times or between the exposure and depuration periods. The major metabolite of 2-CSA, 2-chloro-4-hydroxy-3,5-Dimethoxybenzylalcohol (2-CB-OH), was first detected in the bile of some fish sampled after 24 h of exposure, and the mean concentration of 2-CB-OH in the bile increased over the exposure period. The mean concentration (+/- SE) of 2-CB-OH in the bile was strongly influenced by fish feeding status, being 94 +/- 18 ng/ml bile in fish with empty stomachs and undetectable in fish with full stomachs. Bile volume was also influenced by fish feeding status, being greatest in fish with empty stomachs at the time of sampling. Results indicate that the feeding status of fish should be taken into consideration when using biliary metabolites as biomarkers of effluent exposure in the field, and methods to establish this are discussed.
Metabolites of chlorinated syringaldehydes in fish bile as biomarkers of exposure to bleached eucalypt pulp effluents.[Pubmed:8727518]
Ecotoxicol Environ Saf. 1996 Apr;33(3):253-60.
Metabolites of chlorinated phenolic compounds in fish bile have been found to be sensitive biomarkers of bleached pulp mill effluent exposure. Chlorinated syringaldehydes are largely unstudied chlorophenolics found in bleached hardwood effluent. Sand flathead (Platycephalus bassensis), Australian marine fish, were exposed to 100% chlorine dioxide-bleached eucalypt pulp effluent at concentrations of 0.5, 2, and 8% (v/v) for 4 days. Metabolites of 2-chlorosyringaldehyde (2-CSA), the predominant chlorophenolic in this effluent, were measured in the bile. The major metabolite was the conjugate of 2-chloro-4-hydroxy-3,5-dimethoxy-benzylalcohol (2-CB-OH), the reduced product of 2-CSA. 2-CB-OH was found in all fish exposed to diluted effluent and was concentrated in the bile over 1000 times above 2-CSA levels in the effluent. A separate experiment examined the metabolic fate of 2,6-dichlorosyringaldehyde (2,6-DCSA), which is one of the major chlorophenolics in chlorine-bleached eucalypt pulp effluent. Sand flathead were exposed to 2,6-DCSA by intraperitoneal injection at 15 mg/kg or through the water to 0.5, 2, or 8 micrograms/liter for 4 days. Analysis of the bile revealed the major metabolite of 2,6-DCSA to be the conjugate of 2,6-dichloro-4-hydroxy-3,5-Dimethoxybenzylalcohol, which was found in all exposed fish and was concentrated in the bile over 20,000 times above 2,6-DCSA exposure levels. Results reveal that the analysis of metabolites of chlorinated syringaldehydes in fish bile can provide a biomarker of bleached hardwood effluent exposure that is sensitive to low levels of exposure, specific to certain bleaching sequences, and correlates well with exposure concentrations.


