3,3-Dimethylacrylic acidCAS# 541-47-9 |
Quality Control & MSDS
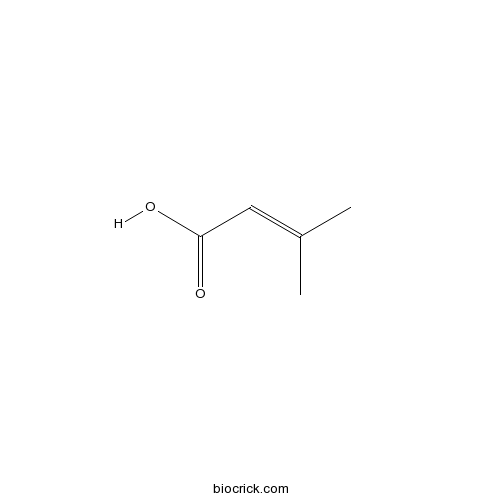
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 541-47-9 | SDF | Download SDF |
| PubChem ID | 10931 | Appearance | Powder |
| Formula | C5H8O2 | M.Wt | 100.12 |
| Type of Compound | Other NPs | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-methylbut-2-enoic acid | ||
| SMILES | CC(=CC(=O)O)C | ||
| Standard InChIKey | YYPNJNDODFVZLE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H8O2/c1-4(2)3-5(6)7/h3H,1-2H3,(H,6,7) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3,3-Dimethylacrylic acid Dilution Calculator

3,3-Dimethylacrylic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.988 mL | 49.9401 mL | 99.8801 mL | 199.7603 mL | 249.7004 mL |
| 5 mM | 1.9976 mL | 9.988 mL | 19.976 mL | 39.9521 mL | 49.9401 mL |
| 10 mM | 0.9988 mL | 4.994 mL | 9.988 mL | 19.976 mL | 24.97 mL |
| 50 mM | 0.1998 mL | 0.9988 mL | 1.9976 mL | 3.9952 mL | 4.994 mL |
| 100 mM | 0.0999 mL | 0.4994 mL | 0.9988 mL | 1.9976 mL | 2.497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Rutamarin
Catalog No.:BCN9204
CAS No.:13164-05-1
- 7α-Galloyloxysweroside
Catalog No.:BCN9203
CAS No.:2222365-76-4
- Viburnumoside
Catalog No.:BCN9202
CAS No.:2222365-74-2
- 12-Hydroxyalbrassitriol
Catalog No.:BCN9201
CAS No.:2193060-23-8
- (Z)-ethyl cinnamate
Catalog No.:BCN9200
CAS No.:4192-77-2
- 5-Hydroxymethyl-2-furoic acid
Catalog No.:BCN9199
CAS No.:6338-41-6
- 7β-Galloyloxysweroside
Catalog No.:BCN9198
CAS No.:2222365-75-3
- Dehydroherbarin
Catalog No.:BCN9197
CAS No.:36379-74-5
- Herbarin
Catalog No.:BCN9196
CAS No.:36379-67-6
- Euglobal Ia1
Catalog No.:BCN9195
CAS No.:77844-93-0
- Filicane-3β,4α,25-triol
Catalog No.:BCN9194
CAS No.:2361548-00-5
- Aflavazole
Catalog No.:BCN9193
CAS No.:2043963-70-6
- O-Demethylstachartin C
Catalog No.:BCN9206
CAS No.:1219361-60-0
- Qingyangshengenin 3-O-α-L-cymaropyranosyl-(1→4)-β-D-oleandropyranosyl-(1→4)-β-D-cymaropyranosyl-(1→4)-β-D-cymaropyranoside
Catalog No.:BCN9207
CAS No.:1808159-02-5
- Chimaphilin
Catalog No.:BCN9208
CAS No.:482-70-2
- Ethyl chlorogenate
Catalog No.:BCN9209
CAS No.:425408-42-0
- Jacaranone
Catalog No.:BCN9210
CAS No.:60263-07-2
- 6-O-(p-Hydroxybenzoyl)glucose
Catalog No.:BCN9211
CAS No.:202337-44-8
- 3"-O-Desmethylspinorhamnoside
Catalog No.:BCN9212
CAS No.:2220243-41-2
- Loroglossin
Catalog No.:BCN9213
CAS No.:58139-22-3
- 3α-Cinnamoyloxy-9β,17-dihydroxy-ent-kaur-15-en-19-oic acid
Catalog No.:BCN9214
CAS No.:2186648-60-0
- 3'-O-Methyltaxifolin
Catalog No.:BCN9215
CAS No.:55812-91-4
- Acutumine
Catalog No.:BCN9216
CAS No.:17088-50-5
- 14β,16β-Dihydroxy-3β-(β-D-glucopyranosyloxy)-5α-bufa-20,22-dienolide
Catalog No.:BCN9217
CAS No.:1323952-04-0
Stable Conversion Chemistry-Based Lithium Metal Batteries Enabled by Hierarchical Multifunctional Polymer Electrolytes with Near-Single Ion Conduction.[Pubmed:30830705]
Angew Chem Int Ed Engl. 2019 Apr 23;58(18):6001-6006.
The low Coulombic efficiency and serious safety issues resulting from uncontrollable dendrite growth have severely impeded the practical applications of lithium (Li) metal anodes. Herein we report a stable quasi-solid-state Li metal battery by employing a hierarchical multifunctional polymer electrolyte (HMPE). This hybrid electrolyte was fabricated via in situ copolymerizing lithium 1-[3-(methacryloyloxy)propylsulfonyl]-1-(trifluoromethanesulfonyl)imide (LiMTFSI) and pentaerythritol tetraacrylate (PETEA) monomers in traditional liquid electrolyte, which is absorbed in a poly(3,3-Dimethylacrylic acid lithium) (PDAALi)-coated glass fiber membrane. The well-designed HMPE simultaneously exhibits high ionic conductivity (2.24x10(-3) S cm(-1) at 25 degrees C), near-single ion conducting behavior (Li ion transference number of 0.75), good mechanical strength and remarkable suppression for Li dendrite growth. More intriguingly, the cation permselective HMPE efficiently prevents the migration of negatively charged iodine (I) species, which provides the as-developed Li-I batteries with high capacity and long cycling stability.
Bis(acetylacetonato) dioxomolybdenum(VI) catalysed rearrangement of methylbutynol in the presence of ultrasound.[Pubmed:11233943]
Ultrason Sonochem. 1999 Mar;6(1-2):89-91.
The molybdenum (VI) catalysed rearrangement of methylbutynol was carried out under the influence of ultrasound (20 kHz). Surprisingly, 2-methylpropene (isobutene) was found as the main product. The formation of isobutene can be explained by rearrangement to prenal, oxidation to 3,3-Dimethylacrylic acid and then decarboxylation. 3,3-Dimethylacrylic acid was decarboxylated in the presence of ultrasound and a catalyst; without a catalyst or without ultrasound (at 50 degrees C) it remained unchanged.


