2B-(SP)Selective GSK-3 phosphopeptide substrate CAS# 186901-17-7 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 186901-17-7 | SDF | Download SDF |
| PubChem ID | 90488714 | Appearance | Powder |
| Formula | C71H123N26O29P | M.Wt | 1835.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
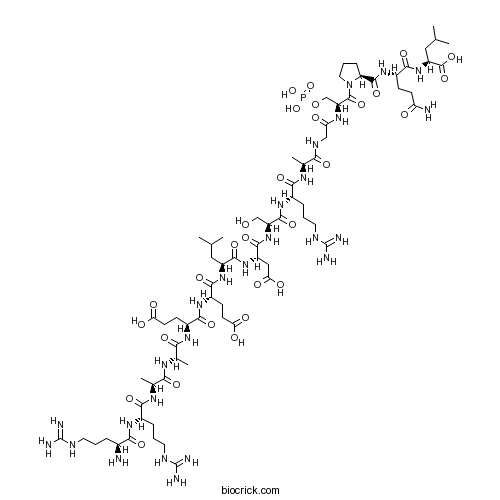
| Sequence | RRAAEELDSRAGSPQL (Modifications: Ser-13 = pSer) | ||
| Chemical Name | (2S)-2-[[(2S)-5-amino-2-[[(2S)-1-[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-5-carbamimidamidopentanoyl]amino]-5-carbamimidamidopentanoyl]amino]propanoyl]amino]propanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-4-methylpentanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]-5-carbamimidamidopentanoyl]amino]propanoyl]amino]acetyl]amino]-3-phosphonooxypropanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-4-methylpentanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CC(=O)O)C(=O)NC(CO)C(=O)NC(CCCNC(=N)N)C(=O)NC(C)C(=O)NCC(=O)NC(COP(=O)(O)O)C(=O)N1CCCC1C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCCNC(=N)N)NC(=O)C(CCCNC(=N)N)N | ||
| Standard InChIKey | LBVCQYQHTKHZRY-APZQELAWSA-N | ||
| Standard InChI | InChI=1S/C71H123N26O29P/c1-32(2)26-43(93-61(114)42(18-21-52(103)104)91-60(113)41(17-20-51(101)102)88-56(109)36(7)84-55(108)35(6)86-58(111)38(13-9-23-81-70(76)77)89-57(110)37(72)12-8-22-80-69(74)75)63(116)94-44(28-53(105)106)64(117)96-46(30-98)65(118)90-39(14-10-24-82-71(78)79)59(112)85-34(5)54(107)83-29-50(100)87-47(31-126-127(123,124)125)67(120)97-25-11-15-48(97)66(119)92-40(16-19-49(73)99)62(115)95-45(68(121)122)27-33(3)4/h32-48,98H,8-31,72H2,1-7H3,(H2,73,99)(H,83,107)(H,84,108)(H,85,112)(H,86,111)(H,87,100)(H,88,109)(H,89,110)(H,90,118)(H,91,113)(H,92,119)(H,93,114)(H,94,116)(H,95,115)(H,96,117)(H,101,102)(H,103,104)(H,105,106)(H,121,122)(H4,74,75,80)(H4,76,77,81)(H4,78,79,82)(H2,123,124,125)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective phosphopeptide substrate for glycogen synthase kinase-3 (GSK-3); derived from the phosphorylation site of the translation factor eIF2B. |

2B-(SP) Dilution Calculator

2B-(SP) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Moxifloxacin HCl
Catalog No.:BCC2507
CAS No.:186826-86-8
- ML 10302 hydrochloride
Catalog No.:BCC7695
CAS No.:186826-17-5
- Ginsenoside Rg5
Catalog No.:BCN3551
CAS No.:186763-78-0
- Alisol A 24-acetate
Catalog No.:BCN2344
CAS No.:18674-16-3
- N6-methyladenosine (m6A)
Catalog No.:BCC6495
CAS No.:1867-73-8
- Ketamine hydrochloride
Catalog No.:BCC5982
CAS No.:1867-66-9
- H-D-Tyr(tBu)-OH
Catalog No.:BCC3137
CAS No.:186698-58-8
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- 2-NBDG
Catalog No.:BCC6530
CAS No.:186689-07-6
- 4-Methylcinnamic acid
Catalog No.:BCN5034
CAS No.:1866-39-3
- Allyl cinnamate
Catalog No.:BCC8812
CAS No.:1866-31-5
- 1,2-Bis(3-indenyl)ethane
Catalog No.:BCC8413
CAS No.:18657-57-3
- Pafuramidine
Catalog No.:BCC1832
CAS No.:186953-56-0
- Sinapine
Catalog No.:BCN1815
CAS No.:18696-26-9
- N,N'-Bis(2-hydroxyethyl)oxamide
Catalog No.:BCC9061
CAS No.:1871-89-2
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- Clauszoline M
Catalog No.:BCN4683
CAS No.:187110-72-1
- Luliconazole
Catalog No.:BCC1711
CAS No.:187164-19-8
- Cyanidin-3-O-rutinoside chloride
Catalog No.:BCN3114
CAS No.:18719-76-1
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Kimcuongin
Catalog No.:BCN7472
CAS No.:1872403-23-0
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Boc-D-FMK
Catalog No.:BCC1128
CAS No.:187389-53-3,634911-80-1
Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B.[Pubmed:22445235]
Colloids Surf B Biointerfaces. 2012 Jun 15;95:23-9.
Biosurfactant-producing bacteria were isolated from terrestrial samples collected in areas contaminated with petroleum compounds. Isolates were screened for biosurfactant production using Cetyl Tri Ammonium Bromide (CTAB)-Methylene blue agar selection medium and the qualitative drop-collapse test. An efficient bacterial strain was selected based on rapid drop collapse activity and highest biosurfactant production. The biochemical characteristics and partial sequenced 16S rRNA gene of isolate, 2B, identified the bacterium as Pseudomonas sp. Five different low cost carbon substrates were evaluated for their effect on biosurfactant production. The maximum biosurfactant synthesis (4.97 g/L) occurred at 96 h when the cells were grown on modified PPGAS medium containing 1% (v/v) molasses at 30 degrees C and 150 rpm. The cell free broth containing the biosurfactant could reduce the surface tension to 30.14 mN/m. The surface active compound showed emulsifying activity against a variety of hydrocarbons and achieved a maximum emulsion index of 84% for sunflower oil. Compositional analysis of the biosurfactant reveals that the extracted biosurfactant was a glycolipid type, which was composed of high percentages of lipid ( approximately 65%, w/w) and carbohydrate ( approximately 32%, w/w). Fourier transform infrared (FT-IR) spectrum of extracted biosurfactant indicates the presence of carboxyl, hydroxyl and methoxyl functional groups. The mass spectra (MS) shows that dirhamnolipid (l-rhamnopyranosyl-l-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoate, Rha-Rha-C(10)-C(10)) was detected in abundance with the predominant congener monorhamnolipid (l-rhamnopyranosyl-beta-hydroxydecanoyl-beta-hydroxydecanoate, Rha-C(10)-C(10)). The crude oil recovery studies using the biosurfactant produced by Pseudomonas sp. 2B suggested its potential application in microbial enhanced oil recovery and bioremediation.
Molecular and genetic analysis of two closely linked genes that encode, respectively, a protein phosphatase 1/2A/2B homolog and a protein kinase homolog in the cyanobacterium Anabaena sp. strain PCC 7120.[Pubmed:9573144]
J Bacteriol. 1998 May;180(10):2616-22.
Reversible protein phosphorylation plays important roles in signal transduction. One gene, prpA, encoding a protein similar to eukaryotic types of phosphoprotein phosphatases PP1, PP2A, and PP2B, was cloned from the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. Interestingly, a eukaryotic-type protein kinase gene, pknE, was found 301 bp downstream of prpA. This unusual genetic arrangement provides the opportunity for study about how the balance between protein phosphorylation and dephosphorylation can regulate cellular activities. Both proteins were overproduced in Escherichia coli and used to raise polyclonal antibodies. Immunodetection and RNA/DNA hybridization experiments suggest that these two genes are unlikely to be coexpressed, despite their close genetic linkage. PrpA is expressed constitutively under different nitrogen conditions, while PknE expression varies according to the nature of the nitrogen source. Inactivation analysis in vivo suggests that PrpA and PknE function to ensure a correct level of phosphorylation of the targets in order to regulate similar biological processes such as heterocyst structure formation and nitrogen fixation.
A new alpha-pyrone from the mangrove endophytic fungus Phomopsis sp. HNY29-2B.[Pubmed:27687677]
Nat Prod Res. 2017 Jan;31(2):124-130.
A new alpha-pyrone derivative, phomopyrone A (1), together with two known compounds (2-3), was isolated from the culture of the mangrove endophytic fungus Phomopsis sp. HNY29-2B. Their structures were determined by detailed analysis of spectroscopic data. The configuration of 1 was further confirmed by X-ray diffraction. All isolated compounds were evaluated for antibacterial and antioxidative activities. Compound 2 exhibited antibacterial activities with minimal inhibition concentration (MIC) values of 25 and 50 muM against Bacillus subtilis and Pseudomonas aeruginosa, and compound 3 showed activities against Staphylococcus aureus and B. subtilis with MIC values of 25 and 50 muM, respectively.
Immunochemical investigations of cytochrome P450 forms/epitopes (CYP1A, 2B, 2E, 3A and 4A) in digestive gland of Mytilus sp.[Pubmed:9972478]
Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998 Nov;121(1-3):361-9.
Western blot analysis of microsomes and partially purified cytochrome P450 (CYP) from digestive gland of Mytilus edulis was carried out using polyclonal antibodies to hepatic Perca fluviatilis CYP1A, Oncorhynchus mykiss CYP3A and rat CYP2B, CYP2E and CYP4A isoforms. Multiple CYP bands were detected in partially purified CYP compared to single bands for microsomes for anti-CYP1A, anti-CYP2B, anti-CYP2E and anti-CYP3A. In contrast, anti-CYP4A showed two distinct bands for both. The apparent molecular weights in kD (mean +/- range or S.D.; n = 2-4) for partially purified CYP were 42.5 +/- 0.5 and 48.1 +/- 0.3 (2 bands, anti-CYP1A); 67.4 +/- 0.7, 52.8 +/- 0.6, 44.5 +/- 2.5 (3 bands, anti-CYP3A); 52.8 +/- 0.7, 48.1 +/- 1.1 and 43.9 +/- 1.1 (3 bands, anti-CYP2B); 52.7 +/- 0.8 and 47.2 +/- 0.2 (2 bands, anti-CYP2E); 50.9 +/- 0.3 and 44.1 +/- 0.2 kD (2 bands, anti-CYP4A). Digestive gland microsomes of Mytilus galloprovincialis from a polluted compared to a clean field site showed higher levels of bands recognised by anti-CYP1A, anti-CYP2E and anti-CYP4A, but not anti-CYP2B and anti-CYP3A (P < 0.05), indicative of independent regulation of different CYP forms. Overall, the apparent molecular weight and field studies indicate at least five different digestive gland CYP forms.
Phosphorylated seryl and threonyl, but not tyrosyl, residues are efficient specificity determinants for GSK-3beta and Shaggy.[Pubmed:10217415]
FEBS Lett. 1999 Apr 1;448(1):86-90.
Glycogen synthase kinase-3 is involved in diverse functions including insulin signalling and development. In a number of substrates, phosphorylation by glycogen synthase kinase-3 is known to require prior phosphorylation at a Ser in the +4 position relative to its own phosphorylation site. Here we have used synthetic peptides derived from a putative glycogen synthase kinase-3 site in the Drosophila translation initiation factor eIF2B epsilon to investigate the efficacy of residues other than Ser(P) as priming residues for glycogen synthase kinase-3beta and its Drosophila homologue Shaggy. Glycogen synthase kinase-3beta phosphorylated peptides with Ser(P) and Thr(P) in the priming position, but peptides with Tyr(P), Thr, Glu or Asp were not phosphorylated. The Vmax for the Thr(P) peptide was three times higher than that of the Ser(P) peptide. These data suggest that glycogen synthase kinase-3 is unique among phosphate-directed kinases. The priming site specificity of Shaggy is similar to that of mammalian glycogen synthase kinase-3beta. This unpredicted efficacy of Thr(P) in the priming position suggests that there may be other unidentified substrates for these kinases.
Peptide substrates suitable for assaying glycogen synthase kinase-3 in crude cell extracts.[Pubmed:9025901]
Anal Biochem. 1997 Jan 1;244(1):16-21.
In this study we describe the characterization and use of new peptide substrates for assaying glycogen synthase kinase-3 (GSK-3) which are based on the sequence around the single GSK-3 phosphorylation site in the translation factor eIF2B. The new peptides offer important advantages over previous substrates, which were based on the sequence around the multiple GSK-3 phosphorylation sites in glycogen synthase (GS), for the assay of GSK-3 in cell extracts. In particular, decreases in GSK-3 activity following, e.g., insulin treatment, are partially or completely masked when the GS-based peptides are used but are readily measured using the new, eIF2B-based, peptides. The new peptides, unlike those based on GS, are therefore suitable for the assay of changes in GSK-3 activity in cell extracts without the need for prior immunoprecipitation or ion-exchange chromatography.


