2-UndecanoneCAS# 112-12-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112-12-9 | SDF | Download SDF |
| PubChem ID | 8163 | Appearance | Liquid |
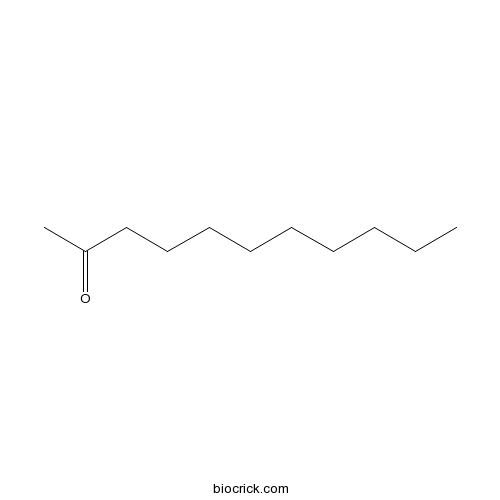
| Formula | C11H22O | M.Wt | 170.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Methyl nonyl ketone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | undecan-2-one | ||
| SMILES | CCCCCCCCCC(=O)C | ||
| Standard InChIKey | KYWIYKKSMDLRDC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H22O/c1-3-4-5-6-7-8-9-10-11(2)12/h3-10H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Undecanone Dilution Calculator

2-Undecanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8723 mL | 29.3617 mL | 58.7234 mL | 117.4467 mL | 146.8084 mL |
| 5 mM | 1.1745 mL | 5.8723 mL | 11.7447 mL | 23.4893 mL | 29.3617 mL |
| 10 mM | 0.5872 mL | 2.9362 mL | 5.8723 mL | 11.7447 mL | 14.6808 mL |
| 50 mM | 0.1174 mL | 0.5872 mL | 1.1745 mL | 2.3489 mL | 2.9362 mL |
| 100 mM | 0.0587 mL | 0.2936 mL | 0.5872 mL | 1.1745 mL | 1.4681 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- Endoxifen
Catalog No.:BCC7761
CAS No.:112093-28-4
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
Direct Growth of Bacteria in Headspace Vials Allows for Screening of Volatiles by Gas Chromatography Mass Spectrometry.[Pubmed:29662472]
Front Microbiol. 2018 Mar 20;9:491.
Bacterially produced volatile organic compounds (VOCs) can modify growth patterns of eukaryotic hosts and competing/cohabiting microbes. These compounds have been implicated in skin disorders and attraction of biting pests. Current methods to detect and characterize VOCs from microbial cultures can be laborious and low-throughput, making it difficult to understand the behavior of microbial populations. In this work we present an efficient method employing gas chromatography/mass spectrometry with autosampling to characterize VOC profiles from solid-phase bacterial cultures. We compare this method to complementary plate-based assays and measure the effects of growth media and incubation temperature on the VOC profiles from a well-studied Pseudomonas aeruginosa PAO1 system. We observe that P. aeruginosa produces longer chain VOCs, such as 2-Undecanone and 2-undecanol in higher amounts at 37 degrees C than 30 degrees C. We demonstrate the throughput of this method by studying VOC profiles from a representative collection of skin bacterial isolates under three parallel growth conditions. We observe differential production of various aldehydes and ketones depending on bacterial strain. This generalizable method will support screening of bacterial populations in a variety of research areas.
Multiple Modes of Nematode Control by Volatiles of Pseudomonas putida 1A00316 from Antarctic Soil against Meloidogyne incognita.[Pubmed:29599753]
Front Microbiol. 2018 Feb 23;9:253.
Pseudomonas putida 1A00316 isolated from Antarctic soil showed nematicidal potential for biological control of Meloidogyne incognita; however, little was known about whether strain 1A00316 could produce volatile organic compounds (VOCs), and if they had potential for use in biological control against M. incognita. In this study, VOCs produced by a culture filtrate of P. putida 1A00316 were evaluated by in vitro experiments in three-compartment Petri dishes and 96-well culture plates. Our results showed that M. incognita juveniles gradually reduced their movement within 24-48 h of incubation with mortality ranging from 6.49 to 86.19%, and mostly stopped action after 72 h. Moreover, egg hatching in culture filtrates of strain 1A00316 was much reduced compared to that in sterile distilled water or culture medium. Volatiles from P. putida 1A00316 analysis carried out by solid-phase micro-extraction gas chromatography-mass spectrometry (SPME-GC/MS) included dimethyl-disulfide, 1-undecene, 2-nonanone, 2-octanone, (Z)-hexen-1-ol acetate, 2-Undecanone, and 1-(ethenyloxy)-octadecane. Of these, dimethyl-disulfide, 2-nonanone, 2-octanone, (Z)-hexen-1-ol acetate, and 2-Undecanone had strong nematicidal activity against M. incognita J2 larvae by direct-contact in 96-well culture plates, and only 2-Undecanone acted as a fumigant. In addition, the seven VOCs inhibited egg hatching of M. incognita both by direct-contact and by fumigation. All of the seven VOCs repelled M. incognita J2 juveniles in 2% water agar Petri plates. These results show that VOCs from strain 1A00316 act on different stages in the development of M. incognita via nematicidal, fumigant, and repellent activities and have potential for development as agents with multiple modes of control of root-knot nematodes.
Differentiation of Rums Produced from Sugar Cane Juice (Rhum Agricole) from Rums Manufactured from Sugar Cane Molasses by a Metabolomics Approach.[Pubmed:29455529]
J Agric Food Chem. 2018 Mar 21;66(11):3038-3045.
A large set of volatiles (a metabolome) was isolated by SAFE distillation from 25 high priced rums prepared from sugar cane juice (SCJ) and 26 high priced rums manufactured from sugar cane molasses (SCM). The volatile fractions were first analyzed by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GCxGC-TOF-MS), and the "comprehensive template matching fingerprinting" was used to extract the entire features present in the respective set of volatile compounds. After raw data pretreatment, chemometrics was used to locate marker compounds. Following, a sparse-partial-least-squares discriminant analysis ( sPLS-DA) and a partial-least-squares discriminant analysis (PLS-DA) were applied to a training data set for creating a model. The model was validated using leave-one-out cross validation and tested over an independent data set to evaluate its predictive power. The characteristic fingerprint resulted in a 100% correct classification of sugar cane juice rums, thus achieving the first aim of locating markers for these higher quality rums. Then, past-processing identification within the discriminant features was done to characterize 12 significant marker compounds as 1-decanol, gamma-dodecalactone, ethyl 3-methylbutanoate, ethyl nonanoate, 3-furancarboxaldehyde, 1-hexanol, beta-ionone, 2- and 3-methylbutanol, methyl decanoate, 3-octanol, and 2-Undecanone. Quantitation of eight selected markers by stable isotope dilution assays confirmed higher concentrations in SCJ compared to SCM and served as the final proof to differentiate both types of spirits.
Chemoreception of botanical nematicides by Meloidogyne incognita and Caenorhabditis elegans.[Pubmed:29708833]
J Environ Sci Health B. 2018 Aug 3;53(8):493-502.
Plant-parasitic nematodes, such as Meloidogyne incognita, cause serious damage to various agricultural crops worldwide, and their control necessitates environmentally safe measures. We have studied the effects of plant secondary metabolites on M. incognita locomotion, as it is an important factor affecting host inoculation inside the soil. We compared the effects to the respective behavioral responses of the model saprophytic nematode Caenorhabditis elegans. The tested botanical nematicides, all reported to be active against Meloidogyne sp. in our previous works, are small molecular weight molecules (acids, alcohols, aldehydes, and ketones). Here, we specifically report on the attractant or repellent properties of trans-anethole, (E,E)-2,4-decadienal, (E)-2-decenal, fosthiazate, and 2-Undecanone. The treatments for both nematode species were made at sublethal concentration levels, namely, 1 mM (
Volatiles emitted by Bacillus sp. BCT9 act as growth modulating agents on Lactuca sativa seedlings.[Pubmed:28754207]
Microbiol Res. 2017 Oct;203:47-56.
Chemical products are applied during horticulture to increase food production, but the environmental problems resulting from these applications have led to a search for more sustainable products. Volatile organic compounds (VOCs) demonstrating plant growth promoter (PGP) activity released by bacterial species have emerged as alternatives, but their effects on Lactuca sativa growth are unknown. In this study, VOCs released by Bacillus sp. BCT9 cultures grown in different media (Methyl Red & Voges Proskauer, Murashige & Skoog and nutrient media) at concentrations of 0.1, 0.2, 0.5 and 0.7 (measured as the absorbance, lambda=600nm) were tested to evaluate their activity as growth inducers of L. sativa after 10days of exposure. Lower concentrations of BCT9 increased root length, and higher concentrations induced shoot length and lateral root length. The dry weight and number of lateral roots increased similarly, independent of concentration, for VOCs produced in all culture media. BCT9 cultures grown in Methyl Red & Voges Proskauer medium as bioactive compounds with or without lanolin. These VOCs increased shoot length, root length and dry weight at low concentrations, independent of the presence of lanolin. Lateral root length increased with the application of 2-nonanone (50ppm) and 2-Undecanone (0.05ppm). Based on these results, the use of bioactive volatiles as growth inducers of horticultural species represents an alternative or complementary strategy.


