2-MethoxystypandroneCAS# 85122-21-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85122-21-0 | SDF | Download SDF |
| PubChem ID | 158739 | Appearance | Red powder |
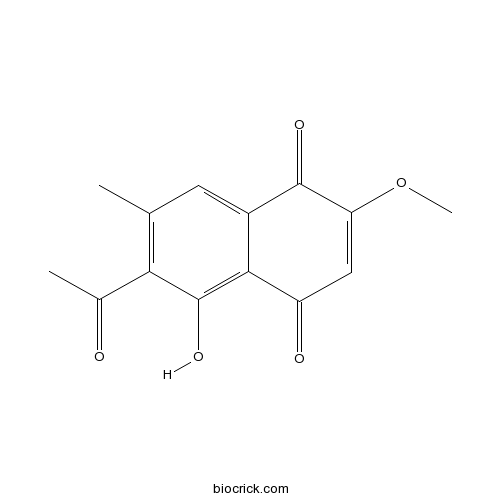
| Formula | C14H12O5 | M.Wt | 260.2 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-acetyl-5-hydroxy-2-methoxy-7-methylnaphthalene-1,4-dione | ||
| SMILES | CC1=C(C(=C2C(=C1)C(=O)C(=CC2=O)OC)O)C(=O)C | ||
| Standard InChIKey | SSHJHOVVYKCJJI-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2-Methoxystypandrone displays an immunomodulatory effect in a cellular model. 2. 2-Methoxystypandrone specifically inhibits JAK and IKKβ kinase activities. 3. 2-Methoxystypandrone blocks inflammatory responses by impairing NF-κB signaling to limit the inflammation and oxidative stress for preservation of BBB integrity. 4. 2-Methoxystypandrone concomitantly promotes neurodevelopmental protein expression and endogenous neurogenesis through inactivation of GSK3β to enhance β-catenin signaling for upexpression of neuroprotective genes and proteins. 5. 2-Methoxystypandrone has anti-osteoclastogenic effect, could reflect the block of RANKL-induced association of TRAF6-TAK1 complexes with consequent decrease of IkappaB-mediated NF-kappaB and mitogen-activated protein kinases-mediated c-Fos activation pathways and suppression of NFATc1 and other gene expression, essential for bone resorption. |
| Targets | JAK | IkB | NF-kB | TNF-α | STAT | NOS | COX | p65 | GSK-3 | Bcl-2/Bax | Wnt/β-catenin | MMP(e.g.TIMP) | IKK |

2-Methoxystypandrone Dilution Calculator

2-Methoxystypandrone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8432 mL | 19.216 mL | 38.432 mL | 76.864 mL | 96.0799 mL |
| 5 mM | 0.7686 mL | 3.8432 mL | 7.6864 mL | 15.3728 mL | 19.216 mL |
| 10 mM | 0.3843 mL | 1.9216 mL | 3.8432 mL | 7.6864 mL | 9.608 mL |
| 50 mM | 0.0769 mL | 0.3843 mL | 0.7686 mL | 1.5373 mL | 1.9216 mL |
| 100 mM | 0.0384 mL | 0.1922 mL | 0.3843 mL | 0.7686 mL | 0.9608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- THIP hydrochloride
Catalog No.:BCC6803
CAS No.:85118-33-8
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Cinchonain IIa
Catalog No.:BCN7716
CAS No.:85081-23-8
- NBI-98782
Catalog No.:BCC4277
CAS No.:85081-18-1
- BX517(PDK1 inhibitor2)
Catalog No.:BCC6391
CAS No.:850717-64-5
- GSK269962A
Catalog No.:BCC5178
CAS No.:850664-21-0
- Alogliptin Benzoate
Catalog No.:BCC1341
CAS No.:850649-62-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Apigenin 5-O-neohesperidoside
Catalog No.:BCN6840
CAS No.:850630-40-9
- Norviburtinal
Catalog No.:BCN4399
CAS No.:85051-41-8
- Fmoc-Arg(Tos)-ol
Catalog No.:BCC2588
CAS No.:850330-29-9
- PF9 tetrasodium salt
Catalog No.:BCC7854
CAS No.:851265-78-6
- Phospho-Glycogen Synthase Peptide-2 (substrate)
Catalog No.:BCC5747
CAS No.:851366-97-7
- (S)-4-Carboxy-3-hydroxyphenylglycine
Catalog No.:BCC6600
CAS No.:85148-82-9
- Curculigoside C
Catalog No.:BCN3696
CAS No.:851713-74-1
- OC000459
Catalog No.:BCC4507
CAS No.:851723-84-7, 950688-14-9 (sodium salt)
- PF 514273
Catalog No.:BCC7746
CAS No.:851728-60-4
- 3-(3-Chloropropyl)-1,3-dihydro-7,8-dimethoxy-2H-3-benzazepin-2-one
Catalog No.:BCC8587
CAS No.:85175-59-3
- ADX-47273
Catalog No.:BCC4598
CAS No.:851881-60-2
- RuBi-4AP
Catalog No.:BCC6044
CAS No.:851956-02-0
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
- BAPTA
Catalog No.:BCC7483
CAS No.:85233-19-8
- 6-Methyl-7-O-methylaromadendrin
Catalog No.:BCN4010
CAS No.:852385-13-8
Discovery, total synthesis, HRV 3C-protease inhibitory activity, and structure-activity relationships of 2-methoxystypandrone and its analogues.[Pubmed:11720861]
Bioorg Med Chem Lett. 2001 Dec 17;11(24):3143-6.
2-Methoxystypandrone, a naphthoquinone, was isolated from a Chinese herb Polygonum cuspidatum by bioassay guided fractionation using HRV 3C-protease assay. It showed an IC(50) value of 4.6 microM and is moderately selective. A new 10-step, total synthesis of 2-Methoxystypandrone was accomplished in 45% overall yield using a Diels-Alder approach. Several analogues of this compound were prepared. Isolation, synthesis and HRV 3C-protease structure-activity relationships of these compounds have been described.
2-Methoxystypandrone represses RANKL-mediated osteoclastogenesis by down-regulating formation of TRAF6-TAK1 signalling complexes.[Pubmed:20735418]
Br J Pharmacol. 2010 Sep;161(2):321-35.
BACKGROUND AND PURPOSE: 2-Methoxystypandrone (2-MS) is a naphthoquinone isolated from Polygonum cuspidatum, a Chinese herb used to treat bone diseases. Here we have determined whether 2-MS antagonised osteoclast development and bone resorption. EXPERIMENTAL APPROACH: RAW264.7 cells were treated with receptor activator of nuclear factor kappaB (NF-kappaB) ligand (RANKL) to induce differentiation into osteoclasts. RT-PCR and Western blot were used to analyse osteoclast-associated gene expression and signalling pathways. KEY RESULTS: The number of multinuclear osteoclasts, actin rings and resorption pit formation were markedly inhibited by 2-MS, targeting osteoclast differentiation at an early stage and without significant cytotoxicity. The anti-resorption effect of 2-MS was accompanied by decreasing dendritic cell-specific transmembrane protein and matrix metalloproteinase-9 (MMP-9) mRNA expression. RANKL-increased MMP-9 gelatinolytic activity was also attenuated by concurrent, but not by subsequent addition of 2-MS. 2-MS markedly inhibited not only the RANKL-triggered nuclear translocations of NF-kappaB, c-Fos and nuclear factor of activated T cells c1 (NFATc1), but also the subsequent NFATc1 induction. Degradation of IkappaB and phosphorylation of mitogen-activated protein kinases were also suppressed. RANKL facilitated the formation of signaling complexes of tumour necrosis factor receptor-associated factor 6 and transforming growth factor beta-activated kinase 1 (TRAF6-TAK1), important for osteoclastogenesis and formation of such signalling complexes was prevented by 2-MS. CONCLUSIONS AND IMPLICATIONS: The anti-osteoclastogenic effects of 2-MS could reflect the block of RANKL-induced association of TRAF6-TAK1 complexes with consequent decrease of IkappaB-mediated NF-kappaB and mitogen-activated protein kinases-mediated c-Fos activation pathways and suppression of NFATc1 and other gene expression, essential for bone resorption.
2-Methoxystypandrone ameliorates brain function through preserving BBB integrity and promoting neurogenesis in mice with acute ischemic stroke.[Pubmed:24342702]
Biochem Pharmacol. 2014 Feb 1;87(3):502-14.
2-Methoxystypandrone (2-MS), a naphthoquinone, has been shown to display an immunomodulatory effect in a cellular model. To explore whether 2-MS could protect mice against cerebral ischemic/reperfusion (I/R)-induced brain injury, we evaluated 2-MS's protective effects on an acute ischemic stroke by inducing a middle cerebral artery occlusion/reperfusion (MCAO) injury in murine model. Treatment of mice that have undergone I/R injury with 2-MS (10-100 mug/kg, i.v.) at 2 h after MCAO enhanced survival rate and ameliorated neurological deficits, brain infarction, neural dysfunction and massive oxidative stress, due to an enormous production of free radicals and breakdown of blood-brain barrier (BBB) by I/R injury; this primarily occurred with extensive infiltration of CD11b-positive inflammatory cells and upexpression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 and p65 nuclear factor-kappa B (NF-kappaB). All of these pathological changes were diminished by 2-MS; 2-MS also intensively limited cortical infarction and promoted upexpression of neurodevelopmental genes near peri-infarct cortex and endogenous neurogenesis near subgranular zone of hippocampal dentate gyrus and the subventricular zone, most possibly by inactivation of GSK3beta which in turn upregulating beta-catenin, Bcl-2 adam11 and adamts20. We conclude that 2-MS blocks inflammatory responses by impairing NF-kappaB signaling to limit the inflammation and oxidative stress for preservation of BBB integrity; 2-MS also concomitantly promotes neurodevelopmental protein expression and endogenous neurogenesis through inactivation of GSK3beta to enhance beta-catenin signaling for upexpression of neuroprotective genes and proteins.
2-Methoxystypandrone inhibits signal transducer and activator of transcription 3 and nuclear factor-kappaB signaling by inhibiting Janus kinase 2 and IkappaB kinase.[Pubmed:24450414]
Cancer Sci. 2014 Apr;105(4):473-80.
Constitutive activation of the signal transducer and activator of transcription 3 (STAT3) or the nuclear factor-kappaB (NF-kappaB) pathway occurs frequently in cancer cells and contributes to oncogenesis. The activation of Janus kinase 2 (JAK2) and IkappaB kinase (IKK) are key events in STAT3 and NF-kappaB signaling, respectively. We have identified 2-Methoxystypandrone (2-MS) from a traditional Chinese medicinal herb Polygonum cuspidatum as a novel dual inhibitor of JAK2 and IKK. 2-MS inhibits both interleukin-6-induced and constitutively-activated STAT3, as well as tumor necrosis factor-alpha-induced NF-kappaB activation. 2-MS specifically inhibits JAK and IKKbeta kinase activities but has little effect on activities of other kinases tested. The inhibitory effects of 2-MS on STAT3 and NF-kappaB signaling can be eliminated by DTT or glutathione and can last for 4 h after a pulse treatment. Furthermore, 2-MS inhibits growth and induces death of tumor cells, particularly those with constitutively-activated STAT3 or NF-kappaB signaling. We propose that the natural compound 2-MS, as a potent dual inhibitor of STAT3 and NF-kappaB pathways, is a promising anticancer drug candidate.


