2-MethoxycinnamaldehydeCAS# 60125-24-8 |
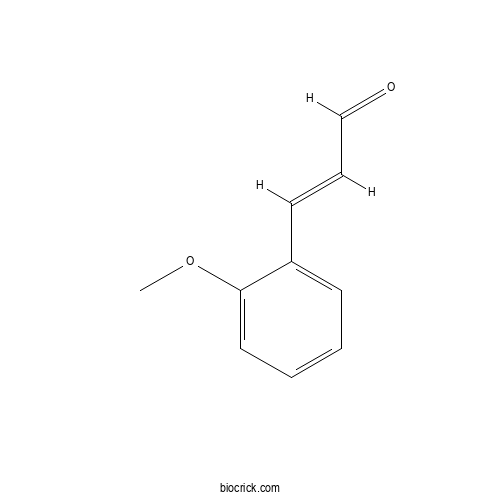
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60125-24-8 | SDF | Download SDF |
| PubChem ID | 641298.0 | Appearance | Powder |
| Formula | C10H10O2 | M.Wt | 162.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (E)-3-(2-Methoxyphenyl)acrylaldehyde;o-Methoxycinnamaldehyde;o-methoxycinnamic aldehyde | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(2-methoxyphenyl)prop-2-enal | ||
| SMILES | COC1=CC=CC=C1C=CC=O | ||
| Standard InChIKey | KKVZAVRSVHUSPL-GQCTYLIASA-N | ||
| Standard InChI | InChI=1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Methoxycinnamaldehyde Dilution Calculator

2-Methoxycinnamaldehyde Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.1656 mL | 30.828 mL | 61.6561 mL | 123.3122 mL | 154.1402 mL |
| 5 mM | 1.2331 mL | 6.1656 mL | 12.3312 mL | 24.6624 mL | 30.828 mL |
| 10 mM | 0.6166 mL | 3.0828 mL | 6.1656 mL | 12.3312 mL | 15.414 mL |
| 50 mM | 0.1233 mL | 0.6166 mL | 1.2331 mL | 2.4662 mL | 3.0828 mL |
| 100 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2331 mL | 1.5414 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3’,5-dihydroxy-2-(4-hydroxybenzyl)3-methoxybibenzyl
Catalog No.:BCX0616
CAS No.:151538-57-7
- 3,4,5-Trimethoxytoluene
Catalog No.:BCX0615
CAS No.:6443-69-2
- Resveratrol-4'-O-β-D-(6''-O-galloy)-glucopyranoside
Catalog No.:BCX0614
CAS No.:64898-03-9
- 3,5-Dimethoxytoluene
Catalog No.:BCX0613
CAS No.:4179-19-5
- Oleanic aldehyde
Catalog No.:BCX0612
CAS No.:17020-22-3
- OJV-VI
Catalog No.:BCX0611
CAS No.:125150-67-6
- Stugeron
Catalog No.:BCX0610
CAS No.:298-57-7
- Arundamine
Catalog No.:BCX0609
CAS No.:475977-53-8
- 5’-inosinic acid
Catalog No.:BCX0608
CAS No.:131-99-7
- Epipinoresinol-4'-O-glucopyranoside
Catalog No.:BCX0607
CAS No.:74983-66-7
- Prosapogenin F
Catalog No.:BCX0606
CAS No.:99365-20-5
- Urolithin B
Catalog No.:BCX0605
CAS No.:1139-83-9
- 11-Oxo-Alpha-Amyrin
Catalog No.:BCX0618
CAS No.:2118-90-3
- Saikogenin F
Catalog No.:BCX0619
CAS No.:14356-59-3
- trans-4-Methoxycinnamic acid
Catalog No.:BCX0620
CAS No.:943-89-5
- 6-Deoxy-D-glucose
Catalog No.:BCX0621
CAS No.:7658-08-4
- 2,3-Dimethoxybenzoic acid
Catalog No.:BCX0622
CAS No.:1521-38-6
- 1-Deoxyforskolin
Catalog No.:BCX0623
CAS No.:72963-77-0
- 4-Methoxy-1,2-benzenediol
Catalog No.:BCX0624
CAS No.:3934-97-2
- Yibeinoside A
Catalog No.:BCX0625
CAS No.:98985-24-1
- γ-sanshool
Catalog No.:BCX0626
CAS No.:78886-65-4
- (+)-Balanophonin
Catalog No.:BCX0627
CAS No.:215319-47-4
- Blestriarene A
Catalog No.:BCX0628
CAS No.:126721-53-7
- Creatinine
Catalog No.:BCX0629
CAS No.:60-27-5
Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.[Pubmed:38019769]
Ann Med. 2023;55(2):2287193.
BACKGROUND: Cinnamomi ramulus (C. ramulus) is frequently employed in the treatment of ankylosing spondylitis (AS). However, the primary constituents, drug targets, and mechanisms of action remain unidentified. METHODS: In this study, various public databases and online tools were employed to gather information on the compounds of C. ramulus, drug targets, and disease targets associated with ankylosing spondylitis. The intersection of drug targets and disease targets was then determined to identify the common targets, which were subsequently used to construct a protein-protein interaction (PPI) network using the STRING database. Network analysis and the analysis of hub genes and major compounds were conducted using Cytoscape software. Furthermore, the Metascape platform was utilized for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Molecular docking studies and immunohistochemical experiments were performed to validate the core targets. RESULTS: The network analysis identified 2-Methoxycinnamaldehyde, cinnamaldehyde, and 2-Hydroxycinnamaldehyde as the major effective compounds present in C. ramulus. The PPI network analysis revealed PTGS2, MMP9, and TLR4 as the most highly correlated targets. GO and KEGG analyses indicated that C. ramulus exerts its therapeutic effects in ankylosing spondylitis through various biological processes, including the response to hormones and peptides, oxidative stress response, and inflammatory response. The main signaling pathways involved were IL-17, TNF, NF-kappa B, and Toll-like receptor pathways. Molecular docking analysis confirmed the strong affinity between the key compounds and the core targets. Additionally, immunohistochemical analysis demonstrated an up-regulation of PTGS2, MMP9, and TLR4 levels in ankylosing spondylitis. CONCLUSIONS: This study provides insights into the effective compounds, core targets, and potential mechanisms of action of C. ramulus in the treatment of ankylosing spondylitis. These findings establish a solid groundwork for future fundamental research in this field.
Evaluation of cinnamaldehyde derivatives as potential protective agents against oxidative-stress induced myotube atrophy using chemical, biological and computational analysis.[Pubmed:37354662]
Bioorg Chem. 2023 Oct;139:106661.
Skeletal muscle atrophy, associated with increased morbidity, mortality and poor quality of life, is a metabolic disorder with no FDA approved drug. Oxidative stress is one of the key mediators of atrophy that influences various cell signaling molecules. The goal of this study is to identify potential antioxidant agents that could be used to treat atrophy. In this study in vitro and in situ screening of different cinnamaldehyde (CNA) derivatives for their antioxidant effects was done along with computational analysis to understand the relationship between their chemical structure and biological activity. Data show that 2-hydroxycinnamaldehyde (2HCNA) worked better than other CNA analogues at physiological pH, while 4-Fluoro-2-Methoxycinnamaldehyde (4FoCNA) showed the maximum antioxidant activity under acidic conditions. However, these derivatives (2HCNA and 4FoCNA) were found to be toxic to the cultured myotubes (mature myofiber) under both physiological and pathophysiological conditions. Immunofluorescence, bright-field microscopic and biochemical studies conducted using live C2C12 cells showed that pre-incubation with other CNA analogues i.e. 2-Methoxycinnamaldehyde (2MeCNA) and 2-benzyloxycinnamaldehyde (2BzCNA) not only maintained the normal morphology of myotubes but also protected them from H2O2-induced atrophy. These compounds (2MeCNA and 2BzCNA) showed higher stability and antioxidant potential, as indicated by computer simulation data analyzed by Density Functional Theory (DFT) based molecular modeling. Overall, the chemical, biological, and computational studies reveal the therapeutic potential of CNA analogues (BzCNA and MeCNA) against oxidative-stress induced muscle atrophy in C2C12 cells.
Attraction of second-stage juveniles of Meloidogyne species to fluopyram.[Pubmed:36905608]
Pest Manag Sci. 2023 Aug;79(8):2696-2703.
BACKGROUND: Several benzenoid aromatic compounds were found to attract second-stage juveniles (J2) of Meloidogyne species in previous studies. Here, the attraction of Meloidogyne J2 to the nematicides fluopyram and fluensulfone, with and without aromatic attractants, was evaluated on agar plates and in sand. RESULTS: Fluensulfone mixed with 2-methoxybenzaldehyde, carvacrol, trans-cinnamic acid, and 2-Methoxycinnamaldehyde, attracted Meloidogyne javanica J2 on an agar plate, whereas fluensulfone alone did not. In contrast, fluopyram alone attracted J2 of M. javanica, Meloidogyne hapla, and Meloidogyne marylandi, although higher numbers of M. javanica J2 were attracted to the nematicide with the aromatic compounds. Trap tubes loaded with 1 and 2 mug fluopyram attracted M. javanica, Meloidogyne incognita, M. hapla, and M. marylandi J2 in the sand. Fluopyram-treated tubes attracted 4.4-6.3 times higher numbers of M. javanica and M. marylandi J2 than fluensulfone. Potassium nitrate (KNO(3) ), a Meloidogyne J2 repellent, did not abolish fluopyram's attractiveness to M. marylandi. These results indicate that high numbers of Meloidogyne J2 near fluopyram on an agar plate or in sand are caused by the attractiveness of the nematicide and not by the accumulation of dead J2 after their random encounter with the nematicide. CONCLUSION: Aromatic attractants have the potential to attract Meloidogyne J2 to nematicides; however, fluopyram itself was attractive to Meloidogyne J2. The attractiveness of fluopyram to Meloidogyne J2 might contribute to its high control efficacy, and elucidation of the attraction mechanism could be useful for nematode-control strategies. (c) 2023 Society of Chemical Industry.
Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.[Pubmed:35875567]
Front Microbiol. 2022 Jul 7;13:864246.
Methicillin-resistant Staphylococcus epidermidis (MRSE) is one of the most commonly found pathogens that may cause uncontrollable infections in immunocompromised and hospitalized patients. Compounds isolated from cinnamon such as cinnamaldehyde and cinnamic acid showed promising anti-oxidant, anti-tumor, and immunoregulatory effects; more importantly, these compounds also possess promising broad-spectrum antibacterial activity. In this study, the potential antibacterial activity of 2-Methoxycinnamaldehyde (MCA), another compound in cinnamon, against MRSE was investigated. Combining the broth microdilution test, live/dead assay, and biofilm formation assay, we found MCA was able to inhibit the proliferation, as well as the biofilm formation of MRSE, indicating MCA could not only affect the growth of MRSE but also inhibit the pathogenic potential of this bacterium. Additionally, the results of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) demonstrated that MCA caused morphological changes and the leakage of DNA, RNA, and cellular contents of MRSE. Due to the close relationship between cell wall synthesis, ROS formation, and cell metabolism, the ROS level and metabolic profile of MRSE were explored. Our study showed MCA significantly increased the ROS production in MRSE, and the following metabolomics analysis showed that the increased ROS production may partially be due to the increased metabolic flux through the TCA cycle. In addition, we noticed the metabolic flux through the pentose phosphate pathway (PPP) was upregulated accompanied by elevated ROS production. Therefore, the alterations in cell metabolism and increased ROS production could lead to the damage of the cell wall, which in turn decreased the proliferation of MRSE. In conclusion, MCA seemed to be a promising alternative antimicrobial agent to control MRSE infections.
NRF2 activation by 2-methoxycinnamaldehyde attenuates inflammatory responses in macrophages via enhancing autophagy flux.[Pubmed:35725014]
BMB Rep. 2022 Aug;55(8):407-412.
A well-controlled inflammatory response is crucial for the recovery from injury and maintenance of tissue homeostasis. The anti-inflammatory response of 2-Methoxycinnamaldehyde (2-MCA), a natural compound derived from cinnamon, has been studied; however, the underlying mechanism on macrophage has not been fully elucidated. In this study, LPS-stimulated production of TNF-alpha and NO was reduced by 2-MCA in macrophages. 2-MCA significantly activated the NRF2 pathway, and expression levels of autophagy-associated proteins in macrophages, including LC3 and P62, were enhanced via NRF2 activation regardless of LPS treatment, suggesting the occurrence of 2-MCA-mediated autophagy. Moreover, evaluation of autophagy flux using luciferase-conjugated LC3 revealed that incremental LC3 and P62 levels are coupled to enhanced autophagy flux. Finally, reduced expression levels of TNF-alpha and NOS2 by 2-MCA were reversed by autophagy inhibitors, such as bafilomycin A1 and NH4Cl, in LPS-stimulated macrophages. In conclusion, 2-MCA enhances autophagy flux in macrophages via NRF2 activation and consequently reduces LPS-induced inflammation. [BMB Reports 2022; 55(8): 407-412].
Metabonomics Study on Naotaifang Extract Alleviating Neuronal Apoptosis after Cerebral Ischemia-Reperfusion Injury.[Pubmed:35321499]
Evid Based Complement Alternat Med. 2022 Mar 14;2022:2112433.
Naotaifang extract (NTE) is a clinically effective traditional Chinese medicine compound for cerebral ischemia-reperfusion injury. Although NTE can achieve neuroprotective function through different mechanisms, the pharmacodynamic substances of NTE corresponding to these mechanisms have rarely been reported. Alleviating or inhibiting neuronal apoptosis is an important way to achieve neuroprotection. Accordingly, this study has evaluated the effects of NTE on alleviating neuronal apoptosis after cerebral ischemia-reperfusion injury from two levels of cells and tissues. Meanwhile, the serum pharmacochemistry of NTE was analyzed by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) with the guidance of Chinmedomics. The results included three aspects: (1) NTE could significantly alleviate neuronal apoptosis caused by in vitro cellular models and in vivo animal models; (2) a total of 21 serum differential metabolites was discovered, including adenosine, inosine, ferulic acid, calycosin, salidroside, 6-gingerol, 2-Methoxycinnamaldehyde, and so on; (3) the metabolic pathway regulated by NTE was mainly purine metabolism. From these results, it can be concluded that alleviating neuronal apoptosis by NTE after cerebral ischemia-reperfusion injury is one of the important mechanisms to achieve neuroprotection. The pharmacodynamic substances of NTE for alleviating neuronal apoptosis on the one hand are related to components directly absorbed into blood, such as ferulic acid, calycosin, salidroside, 6-gingerol, and 2-Methoxycinnamaldehyde and on the other hand are also closely linked to its indirect regulation of purine metabolism in the body to produce adenosine and inosine. Therefore, our research not only identified the main pharmacodynamic substances of NTE that alleviated neuronal apoptosis but also provided a methodological reference for studying other neuroprotective effects of NTE.
Screening the Q-markers of TCMs from RA rat plasma using UHPLC-QTOF/MS technique for the comprehensive evaluation of Wu-Wei-Wen-Tong Capsule.[Pubmed:33764633]
J Mass Spectrom. 2021 May;56(5):e4711.
The appropriate selection of quality marker (Q-marker) for performing the comprehensive quality evaluation of traditional Chinese medicines (TCMs) has much more significance. Wu-Wei-Wen-Tong Capsule (WWWTC), a TCMs prescription, is mainly utilized to treat rheumatoid arthritis (RA) in China. However, the comprehensive quality control for WWWTC has not been achieved because of lacking system analysis for the Q-marker. In this study, a dual wavelength, 203 and 270 nm, was selected based on the feature of 15 Q-markers, and a reliable UHPLC-UV fingerprinting approach was established, achieving the comprehensive quality evaluation of WWWTC. First, we identified 91 prototypes in rat plasma after administering a set amount of WWWTC by using UHPLC-QTOF/MS technique and selected them as the candidate Q-markers. Next, based on the "five principles" of Q-marker selection, 15 absorbed components among them including coumarin, cinnamic acid, cinnamaldehyde, cinnamic alcohol, and 2-Methoxycinnamaldehyde derived from Monarch medicine of Cmnamomi Mmulus; epimedin C, icariin, baohuoside I, and anhydroicaritin derived from Monarch medicine Epimedii Folium; germacrone, the sesquiterpene compound in Minister medicine Rhizoma Wenyujin Concisum; pachymic acid, the tetracyclic triterpenoid acids in Assistant medicine Poria; baicalin, baicalein, wogonin, and wogonoside in Guide medicine Scutellariae Radix, respectively, were seriously chosen as the Q-markers, indicating preferable pharmacological effect on RA, characterization of transitivity and traceability as well as measurable components in WWWTC. The effective and meaningful strategy displayed a unique perspective for the exploration of Q-markers in the quality evaluation and further ensured efficacy and safety of the TCMs.
Network pharmacology-based research uncovers cold resistance and thermogenesis mechanism of Cinnamomum cassia.[Pubmed:33388379]
Fitoterapia. 2021 Mar;149:104824.
BACKGROUND: Cinnamomum cassia (L.) J.Presl (Cinnamon) was known as a kind of hot herb, improved circulation and warmed the body. However, the active components and mechanisms of dispelling cold remain unknown. METHODS: The effects of several Chinses herbs on thermogenesis were evaluated on body temperature and activation of brown adipose tissue. After confirming the effect, the components of cinnamon were identified using HPLC-Q-TOF/MS and screened with databases. The targets of components were obtained with TCMSP, SymMap, Swiss and STITCH databases. Thermogenesis genes were predicted with DisGeNET and GeneCards databases. The protein-protein interaction network was constructed with Cytoscape 3.7.1 software. GO enrichment analysis was accomplished with STRING databases. KEGG pathway analysis was established with Omicshare tools. The top 20 targets for four compounds were obtained according to the number of edges of PPI network. In addition, the network results were verified with experimental research for the effects of extracts and major compounds. RESULTS: Cinnamon extract significantly upregulated the body temperature during cold exposure.121 components were identified in HPLC-Q-TOF/MS. Among them, 60 compounds were included in the databases. 116 targets were obtained for the compounds, and 41 genes were related to thermogenesis. The network results revealed that 27 active ingredients and 39 target genes. Through the KEGG analysis, the top 3 pathways were PPAR signaling pathway, AMPK signaling pathway, thermogenesis pathway. The thermogenic protein PPARgamma, UCP1 and PGC1-alpha was included in the critical targets of four major compounds. The three major compounds increased the lipid consumption and activated the brown adipocyte. They also upregulated the expression of UCP1, PGC1-alpha and pHSL, especially 2-Methoxycinnamaldehyde was confirmed the effect for the first time. Furthermore, cinnamaldehyde and cinnamon extract activated the expression of TRPA1 on DRG cells. CONCLUSION: The mechanisms of cinnamon on cold resistance were investigated with network pharmacology and experiment validation. This work provided research direction to support the traditional applications of thermogenesis.
Integrated computational approach toward discovery of multi-targeted natural products from Thumbai (Leucas aspera) for attuning NKT cells.[Pubmed:33179569]
J Biomol Struct Dyn. 2022 Apr;40(7):2893-2907.
A multi-omics-based approach targeting the plant-based natural products from Thumbai (Leucas aspera), an important yet untapped potential source of many therapeutic agents for myriads of immunological conditions and genetic disorders, was conceptualized to reconnoiter its potential biomedical application. A library of 79 compounds from this plant was created, out of which 9 compounds qualified the pharmacokinetics parameters. Reverse pharmacophore technique for target fishing of the screened compounds was executed through which renin receptor (ATP6AP2) and thymidylate kinase (DTYMK) were identified as potential targets. Network biology approaches were used to comprehend and validate the functional, biochemical and clinical relevance of the targets. The target-ligand interaction and subsequent stability parameters at molecular scale were investigated using multiple strategies including molecular modeling, pharmacophore approaches and molecular dynamics simulation. Herein, isololiolide and 4-hydroxy-2-Methoxycinnamaldehyde were substantiated as the lead molecules exhibiting comparatively the best binding affinity against the two putative protein targets. These natural lead products from L. aspera and the combinatorial effects may have plausible medical applications in a wide variety of neurodegenerative, genetic and developmental disorders. The lead molecules also exhibit promising alternative in diagnostics and therapeutics through immuno-modulation targeting natural killer T-cell function in transplantation-related pathogenesis, autoimmune and other immunological disorders.Communicated by Ramaswamy H. Sarma.
Mechanisms of Herb-Drug Interactions Involving Cinnamon and CYP2A6: Focus on Time-Dependent Inhibition by Cinnamaldehyde and 2-Methoxycinnamaldehyde.[Pubmed:32788161]
Drug Metab Dispos. 2020 Oct;48(10):1028-1043.
Information is scarce regarding pharmacokinetic-based herb-drug interactions (HDI) with trans-cinnamaldehyde (CA) and 2-Methoxycinnamaldehyde (MCA), components of cinnamon. Given the presence of cinnamon in food and herbal treatments for various diseases, HDIs involving the CYP2A6 substrates nicotine and letrozole with MCA (K(S) = 1.58 microM; Hill slope = 1.16) and CA were investigated. The time-dependent inhibition (TDI) by MCA and CA of CYP2A6-mediated nicotine metabolism is a complex process involving multiple mechanisms. Molecular dynamic simulations showed that CYP2A6's active site accommodates two dynamic ligands. The preferred binding orientations for MCA and CA were consistent with the observed metabolism: epoxidation, O-demethylation, and aromatic hydroxylation of MCA and cinnamic acid formation from CA. The percent remaining activity plots for TDI by MCA and CA were curved, and they were analyzed with a numerical method using models of varying complexity. The best-fit models support multiple inactivator binding, inhibitor depletion, and partial inactivation. Deconvoluted mass spectra indicated that MCA and CA modified CYP2A6 apoprotein with mass additions of 156.79 (142.54-171.04) and 132.67 (123.37-141.98), respectively, and it was unaffected by glutathione. Heme degradation was observed in the presence of MCA (48.5% +/- 13.4% loss; detected by liquid chromatography-tandem mass spectrometry). In the absence of clinical data, HDI predictions were made for nicotine and letrozole using inhibition parameters from the best-fit TDI models and parameters scaled from rats. Predicted area under the concentration-time curve fold changes were 4.29 (CA-nicotine), 4.92 (CA-letrozole), 4.35 (MCA-nicotine), and 5.00 (MCA-letrozole). These findings suggest that extensive exposure to cinnamon (corresponding to approximately 275 mg CA) would lead to noteworthy interactions. SIGNIFICANCE STATEMENT: Human exposure to cinnamon is common because of its presence in food and cinnamon-based herbal treatments. Little is known about the risk for cinnamaldehyde and methoxycinnamaldehyde, two components of cinnamon, to interact with drugs that are eliminated by CYP2A6-mediated metabolism. The interactions with CYP2A6 are complex, involving multiple-ligand binding, time-dependent inhibition of nicotine metabolism, heme degradation, and apoprotein modification. An herb-drug interaction prediction suggests that extensive exposure to cinnamon would lead to noteworthy interactions with nicotine.


