2-Hydroxy-1,8-cineoleCAS# 60761-00-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60761-00-4 | SDF | Download SDF |
| PubChem ID | 109010 | Appearance | Oil |
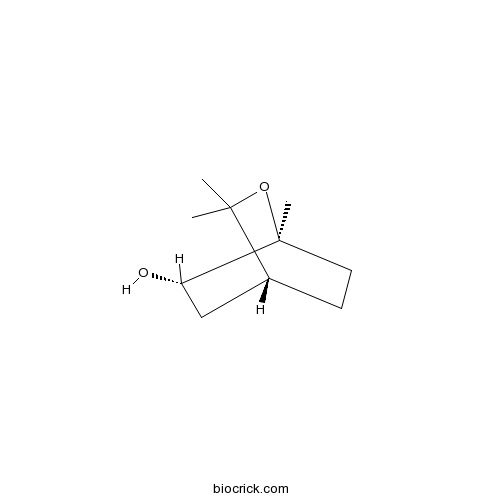
| Formula | C10H18O2 | M.Wt | 170.3 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4S,5S)-2,2,4-trimethyl-3-oxabicyclo[2.2.2]octan-5-ol | ||
| SMILES | CC1(C2CCC(O1)(C(C2)O)C)C | ||
| Standard InChIKey | YVCUGZBVCHODNB-WEDXCCLWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2-endo-Hydroxy-1,8-cineole shows antimicrobial and bactericidal activities against against test bacteria (Staphylococcus aureus, Escherichia coli, and Pseudomonas fluorescens) . |
| Targets | Antifection |

2-Hydroxy-1,8-cineole Dilution Calculator

2-Hydroxy-1,8-cineole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.872 mL | 29.36 mL | 58.7199 mL | 117.4398 mL | 146.7998 mL |
| 5 mM | 1.1744 mL | 5.872 mL | 11.744 mL | 23.488 mL | 29.36 mL |
| 10 mM | 0.5872 mL | 2.936 mL | 5.872 mL | 11.744 mL | 14.68 mL |
| 50 mM | 0.1174 mL | 0.5872 mL | 1.1744 mL | 2.3488 mL | 2.936 mL |
| 100 mM | 0.0587 mL | 0.2936 mL | 0.5872 mL | 1.1744 mL | 1.468 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Canthin-6-one N-oxide
Catalog No.:BCN2992
CAS No.:60755-87-5
- AZ 10606120 dihydrochloride
Catalog No.:BCC6005
CAS No.:607378-18-7
- SB 772077B dihydrochloride
Catalog No.:BCC6116
CAS No.:607373-46-6
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
- Myristicin
Catalog No.:BCN2730
CAS No.:607-91-0
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- FC 131
Catalog No.:BCC7917
CAS No.:606968-52-9
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- ent-Kauran-17,19-dioic acid
Catalog No.:BCN4122
CAS No.:60761-79-7
- Meloside A
Catalog No.:BCN2278
CAS No.:60767-80-8
- Ethyl 4-methoxysalicylate
Catalog No.:BCN3499
CAS No.:35031-00-6
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- SB742457
Catalog No.:BCC5058
CAS No.:607742-69-8
- Quercetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN7821
CAS No.:60778-02-1
- Berbamine hydrochloride
Catalog No.:BCN2400
CAS No.:6078-17-7
- Norbraylin
Catalog No.:BCN4124
CAS No.:60796-64-7
- 5,7,8-Trimethoxycoumarin
Catalog No.:BCN4125
CAS No.:60796-65-8
- Dulcitol
Catalog No.:BCN8153
CAS No.:608-66-2
- Sinomenine HCl
Catalog No.:BCN6318
CAS No.:6080-33-7
- Lithium Citrate
Catalog No.:BCC3804
CAS No.:6080-58-6
Antimicrobial and bactericidal activities of esters of 2-endo-hydroxy-1,8-cineole as new aroma chemicals.[Pubmed:12033822]
J Agric Food Chem. 2002 Jun 5;50(12):3522-6.
Fourteen kinds of alkyl esters of 2-endo-hydroxy-1,8-cineole were synthesized, with yields of 57.8-98.0%. Each ester had a characteristic and unique odor. Especially, the tert-butyl acetate of 2-endo-hydroxy-1,8-cineole had the most interesting odor of all the synthetic esters. The antimicrobial and bactericidal activities of these synthetic esters against test bacteria (Staphylococcus aureus, Escherichia coli, and Pseudomonas fluorescens) were examined using the broth dilution method. As a result, the tert-butyl acetate of 2-endo-hydroxy-1,8-cineole showed the highest antimicrobial and bactericidal activities against all kinds of the test bacteria.
Quantification of 1,8-cineole and of its metabolites in humans using stable isotope dilution assays.[Pubmed:20425757]
Mol Nutr Food Res. 2010 Oct;54(10):1515-29.
The metabolism of 1,8-cineole after ingestion of sage tea was studied. After application of the tea, the metabolites 2-Hydroxy-1,8-cineole, 3-hydroxy-1,8-cineole, 9-hydroxy-1,8-cineole and, for the first time in humans, 7-hydroxy-1,8-cineole were identified in plasma and urine of one volunteer. For quantitation of these metabolites and the parent compound, stable isotope dilution assays were developed after synthesis of [(2)H(3)]-1,8-cineole, [9/10-(2)H(3)]-2-Hydroxy-1,8-cineole and [(13)C,(2)H(2)]-9-hydroxy-1,8-cineole as internal standards. Using these standards, we quantified 1,8-cineole by solid phase microextraction GC-MS and the hydroxyl-1,8-cineoles by LC-MS/MS after deconjugation in blood and urine of the volunteer. After consumption of 1.02 mg 1,8-cineole (19 mug/kg bw), the hydroxycineoles along with their parent compound were detectable in the blood plasma of the volunteer under study after liberation from their glucuronides with 2-hydroxycineole being the predominant metabolite at a maximum plasma concentration of 86 nmol/L followed by the 9-hydroxy isomer at a maximum plasma concentration of 33 nmol/L. The parent compound 1,8-cineole showed a low maximum plasma concentration of 19 nmol/L. In urine, 2-hydroxycineole also showed highest contents followed by its 9-isomer. Summing up the urinary excretion over 10 h, 2-hydroxycineole, the 9-isomer, the 3-isomer and the 7-isomer accounted for 20.9, 17.2, 10.6 and 3.8% of the cineole dose, respectively.


