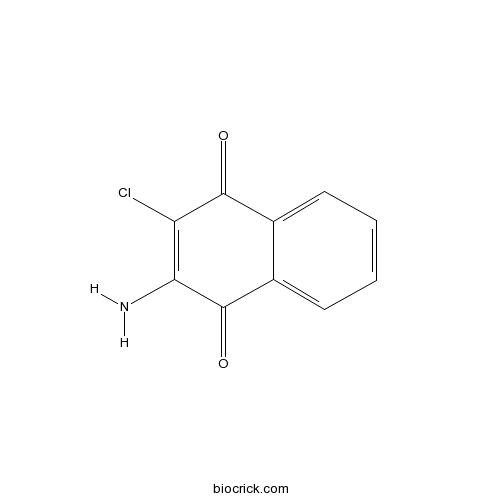
2-Amino-3-chloro-1,4-naphthoquinoneCAS# 2797-51-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2797-51-5 | SDF | Download SDF |
| PubChem ID | 17748 | Appearance | Powder |
| Formula | C10H6ClNO2 | M.Wt | 207.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 250 mg/mL (1204.18 mM; Need ultrasonic) | ||
| Chemical Name | 2-amino-3-chloronaphthalene-1,4-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=C(C2=O)Cl)N | ||
| Standard InChIKey | OBLNWSCLAYSJJR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H6ClNO2/c11-7-8(12)10(14)6-4-2-1-3-5(6)9(7)13/h1-4H,12H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Amino-3-chloro-1,4-naphthoquinone Dilution Calculator

2-Amino-3-chloro-1,4-naphthoquinone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.817 mL | 24.0848 mL | 48.1696 mL | 96.3391 mL | 120.4239 mL |
| 5 mM | 0.9634 mL | 4.817 mL | 9.6339 mL | 19.2678 mL | 24.0848 mL |
| 10 mM | 0.4817 mL | 2.4085 mL | 4.817 mL | 9.6339 mL | 12.0424 mL |
| 50 mM | 0.0963 mL | 0.4817 mL | 0.9634 mL | 1.9268 mL | 2.4085 mL |
| 100 mM | 0.0482 mL | 0.2408 mL | 0.4817 mL | 0.9634 mL | 1.2042 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14-Deoxy-epsilon-caesalpin
Catalog No.:BCN7254
CAS No.:279683-46-4
- H-Glu(OBzl)-OBzl.TosOH
Catalog No.:BCC2928
CAS No.:2791-84-6
- H-Lys(Z)-OMe.HCl
Catalog No.:BCC2988
CAS No.:27894-50-4
- Bis(4-bromophenyl)acetylene
Catalog No.:BCC8882
CAS No.:2789-89-1
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Croceic acid
Catalog No.:BCN2372
CAS No.:27876-94-4
- NH125
Catalog No.:BCC4001
CAS No.:278603-08-0
- Lamiide
Catalog No.:BCN4656
CAS No.:27856-54-8
- Nicergoline
Catalog No.:BCC5214
CAS No.:27848-84-6
- Macrophylline
Catalog No.:BCN1987
CAS No.:27841-97-0
- Loxapine Succinate
Catalog No.:BCC4674
CAS No.:27833-64-3
- Serratenediol diacetate
Catalog No.:BCN5173
CAS No.:27832-84-4
- 1,6-Dibromopyrene
Catalog No.:BCC8428
CAS No.:27973-29-1
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
Cr(VI) reduction and Cr(III) immobilization by Acinetobacter sp. HK-1 with the assistance of a novel quinone/graphene oxide composite.[Pubmed:25296002]
Environ Sci Technol. 2014 Nov 4;48(21):12876-85.
Cr(VI) biotreatment has attracted a substantial amount of interest due to its cost effectiveness and environmental friendliness. However, the slow Cr(VI) bioreduction rate and the formed organo-Cr(III) in solution are bottlenecks for biotechnology application. In this study, a novel strain, Acinetobacter sp. HK-1, capable of reducing Cr(VI) and immobilizing Cr(III) was isolated. Under optimal conditions, the Cr(VI) reduction rate could reach 3.82 mg h(-1) g cell(-1). To improve the Cr(VI) reduction rate, two quinone/graphene oxide composites (Q-GOs) were first prepared via a one-step covalent chemical reaction. The results showed that 2-Amino-3-chloro-1,4-naphthoquinone-GO (NQ-GO) exhibited a better catalytic performance in Cr(VI) reduction compared to 2-aminoanthraquinone-GO. Specifically, in the presence of 50 mg L(-1) NQ-GO, a Cr(VI) removal rate of 190 mg h(-1) g cell(-1), which was the highest rate obtained, was achieved. The increased Cr(VI) reduction rate is mainly the result of NQ-GO significantly increasing the Cr(VI) reduction activity of cell membrane proteins containing dominant Cr(VI) reductases. X-ray photoelectron spectroscopy analysis found that Cr(VI) was reduced to insoluble Cr(III), which was immobilized by glycolipids secreted by strain HK-1. These findings indicate that the application of strain HK-1 and NQ-GO is a promising strategy for enhancing the treatment of Cr(VI)-containing wastewater.
Synthesis and characterization of novel unsymmetrical and symmetrical 3-halo- or 3-methoxy-substituted 2-dibenzoylamino-1,4-naphthoquinone derivatives.[Pubmed:23381023]
Molecules. 2013 Feb 4;18(2):1973-84.
Symmetrical and unsymmetrical 3-halo- or 3-methoxy- substituted 2-dibenzoylamino- 1,4-naphthoquinone analogs were synthesized with an average yield of 45% via sodium hydride promoted bis-acylation of 2-Amino-3-chloro-1,4-naphthoquinone, 2-amino-3-bromo-1,4-naphthoquinone and 2-amino-3-methoxy-1,4-naphthoquinone.
Comprehensive evaluation of a novel nuclear factor-kappaB inhibitor, quinoclamine, by transcriptomic analysis.[Pubmed:19422389]
Br J Pharmacol. 2009 Jul;157(5):746-56.
BACKGROUND AND PURPOSE: The transcription factor nuclear factor-kappaB (NF-kappaB) has been linked to the cell growth, apoptosis and cell cycle progression. NF-kappaB blockade induces apoptosis of cancer cells. Therefore, NF-kappaB is suggested as a potential therapeutic target for cancer. Here, we have evaluated the anti-cancer potential of a novel NF-kappaB inhibitor, quinoclamine (2-Amino-3-chloro-1,4-naphthoquinone). EXPERIMENTAL APPROACH: In a large-scale screening test, we found that quinoclamine was a novel NF-kappaB inhibitor. The global transcriptional profiling of quinoclamine in HepG2 cells was therefore analysed by transcriptomic tools in this study. KEY RESULTS: Quinoclamine suppressed endogenous NF-kappaB activity in HepG2 cells through the inhibition of IkappaB-alpha phosphorylation and p65 translocation. Quinoclamine also inhibited induced NF-kappaB activities in lung and breast cancer cell lines. Quinoclamine-regulated genes interacted with NF-kappaB or its downstream genes by network analysis. Quinoclamine affected the expression levels of genes involved in cell cycle or apoptosis, suggesting that quinoclamine exhibited anti-cancer potential. Furthermore, quinoclamine down-regulated the expressions of UDP glucuronosyltransferase genes involved in phase II drug metabolism, suggesting that quinoclamine might interfere with drug metabolism by slowing down the excretion of drugs. CONCLUSION AND IMPLICATIONS: This study provides a comprehensive evaluation of quinoclamine by transcriptomic analysis. Our findings suggest that quinoclamine is a novel NF-kappaB inhibitor with anti-cancer potential.
Synthesis and MEK1 inhibitory activities of imido-substituted 2-chloro-1,4-naphthoquinones.[Pubmed:12818679]
Bioorg Med Chem. 2003 Jul 17;11(14):3165-70.
Mitogen activated protein kinases are of interest as research tools and as therapeutic target for certain physiological disorders. In this study, we found 2-chloro-3-(N-succinimidyl)-1,4-naphthoquinone 6 to be a selective inhibitor of MEK1 with an IC(50) of 0.38 microM. An open-chain homologue, 10, showed selective cytotoxicity against renal cancer in the NCI in vitro tumor screening. Structure-activity relationship study of eight compounds showed the cyclic imido-substituted chloro-1,4-naphthoquinone as more potent and selective MEK1 inhibitors than the open chain homologues. The imido-substituted chloro-1,4-naphthoquinones were synthesized in a straightforward fashion by refluxing 2-Amino-3-chloro-1,4-naphthoquinone with the appropriate acid chloride or diacyl dichloride.
Aminonaphthoquinones--a novel class of compounds with potent antimalarial activity against Plasmodium falciparum.[Pubmed:11352541]
Pharmacol Res. 2001 Apr;43(4):363-7.
Malaria is a major tropical disease, which kills two million people annually. The population at risk from this disease has increased because of the difficulties in eradicating the mosquito vector in the endemic regions and the emergence and spread of parasite resistance to all the commonly used antimalarials. Since antimalarials are the major arsenal for treatment of the disease, there is an urgent need for newer drugs with novel mechanisms of action, which will be effective against all strains of the parasite. As a part of our anti-infective drug discovery program, we have investigated 18 compounds including several synthetic and natural naphthoquinones as potential antimalarial agents. We have identified aminonaphthoquinones, as a class of antimalarial compounds with antimalarial activity against Plasmodium falciparum. Among these compounds, 2-Amino-3-chloro-1,4-naphthoquinone is the most potent. It had an IC(50)of 0.18 micro M (37.3 ng ml(-1)) against the W2 clone, and is more potent than chloroquine, which had an IC(50)of 0.23 micro M (72 ng ml(-1)). It was also active against the D6 clone. In general, 2-amino-1,4-naphthoquinone analogs and the 4-amino-1,2-napthoquinone analog showed promising antimalarial activity in the bioassay. In contrast, a number of 2-hydroxy-1,4-naphthoquinones and dimeric quinones were less active.
Studies on Effects of Certain Quinones: II. Photosynthetic Incorporation of CO(2) by Chlorella.[Pubmed:16657966]
Plant Physiol. 1972 Mar;49(3):385-7.
The effects of various quinone herbicides and fungicides on the photosynthetic (14)CO(2) fixation and the incorporation of (14)C among the products of photosynthesis in Chlorella pyrenoidosa was investigated. Addition of 30 mum 2,3-dichloro-1,4-naphthoquinone (dichlone), 2-Amino-3-chloro-1,4-naphthoquinone (06K-quinone), or 2,3,5,6-tetrachloro-1,4-benzoquinone (chloranil) inhibited CO(2) fixation, whereas 1,4-benzoquinone had no effect. Treatment with 3 mum or higher concentrations of dichlone, 06K-quinone or 1,4-benzoquinone also produced marked changes in the pattern of (14)C distribution. A noticeable effect was an increase in the proportion of (14)C in sucrose and glycine accompanied by a reduction in (14)C lipids and glutamic acid. These changes appear to occur as a result of shifts in the flow of carbon along various biosynthetic pathways of photosynthetic CO(2) fixation. It is suggested that inactivation of coenzyme A and shortage of reduced triphosphopyridine nucleotide in the quinone-treated cells inhibited the synthesis of lipids and glutamic acid, thereby diverting more carbon into sucrose and glycine.
Studies on Effect of Certain Quinones: I. Electron Transport, Photophosphorylation, and CO(2) Fixation in Isolated Chloroplasts.[Pubmed:16657965]
Plant Physiol. 1972 Mar;49(3):381-4.
The effect of quinone herbicides and fungicides on photosynthetic reactions in isolated spinach (Spinacia oleracea) chloroplasts was investigated. 2,3-Dichloro-1,4-naphthoquinone (dichlone), 2-Amino-3-chloro-1,4-naphthoquinone (06K-quinone), and 2,3,5,6-tetrachloro-1,4-benzoquinone (chloranil) inhibited ferricyanide reduction as well as ATP formation. Benzoquinone had little or no effect on these reactions. The two reactions showed a differential sensitivity to these inhibitors. Dichlone was a strong inhibitor of both photosystems I and II; photosystem I was more sensitive to 06K-quinone than was photosystem II, whereas the reverse was true of chloranil. Chloranil and 06K-quinone inhibited ferricyanide reduction and the coupled photophosphorylation to the same extent, whereas dichlone affected photophosphorylation to a greater extent than the ferricyanide reduction.CO(2) fixation was inhibited by all the quinones to varying degrees. In chloroplasts treated with 06K-quinone or benzoquinone, CO(2) fixation was inhibited to a greater extent than the photoreduction of ferricyanide or ATP formation, indicating the possibility that the two quinones may also inhibit certain reactions in the carbon reduction cycle. The effect of dichlone and chloranil, but not of 06K-quinone, was overcome by the addition of reduced glutathione. The quinones caused an increase in the proportion of (14)C incorporated into 3-phosphoglyceric acid and a reduction in the amount of glycolic acid.


