2,3-Dihydroxypterodontic acidCAS# 185821-32-3 |
Quality Control & MSDS
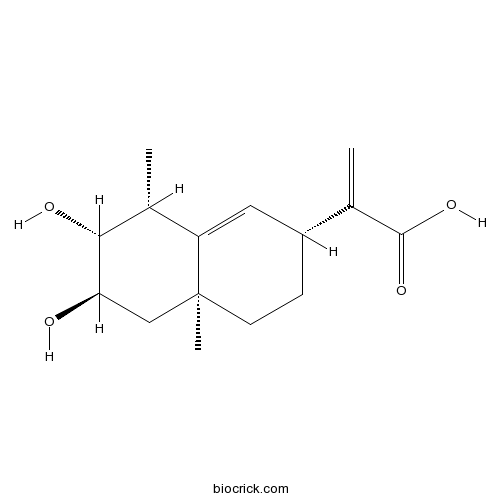
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 185821-32-3 | SDF | Download SDF |
| PubChem ID | 23872152 | Appearance | Cryst. |
| Formula | C15H22O4 | M.Wt | 266.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[(2R,4aS,6R,7R,8R)-6,7-dihydroxy-4a,8-dimethyl-3,4,5,6,7,8-hexahydro-2H-naphthalen-2-yl]prop-2-enoic acid | ||
| SMILES | CC1C(C(CC2(C1=CC(CC2)C(=C)C(=O)O)C)O)O | ||
| Standard InChIKey | MNLUMTSGGLOUCH-WHLPLNIVSA-N | ||
| Standard InChI | InChI=1S/C15H22O4/c1-8(14(18)19)10-4-5-15(3)7-12(16)13(17)9(2)11(15)6-10/h6,9-10,12-13,16-17H,1,4-5,7H2,2-3H3,(H,18,19)/t9-,10-,12-,13-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,3-Dihydroxypterodontic acid Dilution Calculator

2,3-Dihydroxypterodontic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Viroallosecurinine
Catalog No.:BCN6743
CAS No.:1857-30-3
- BMS 961
Catalog No.:BCC7680
CAS No.:185629-22-5
- ONO 2506
Catalog No.:BCC7943
CAS No.:185517-21-9
- Timorsaponin C
Catalog No.:BCN2899
CAS No.:185432-00-2
- Corchoionoside C
Catalog No.:BCN1154
CAS No.:185414-25-9
- AF 12198
Catalog No.:BCC5812
CAS No.:185413-30-3
- O-Acetylethanolamine
Catalog No.:BCN1757
CAS No.:1854-30-4
- TCS 1105
Catalog No.:BCC6087
CAS No.:185391-33-7
- Fmoc-3-(2-Pyridyl)-Alanine
Catalog No.:BCC2568
CAS No.:185379-40-2
- Fmoc-3-(2-Pyridyl)-D-Alanine
Catalog No.:BCC2569
CAS No.:185379-39-9
- (R)-(+)-1,1'-Bi-2-naphthol
Catalog No.:BCC8393
CAS No.:18531-94-7
- GR 46611
Catalog No.:BCC5679
CAS No.:185259-85-2
- Pterodontic acid
Catalog No.:BCN1156
CAS No.:185845-89-0
- Echinulin
Catalog No.:BCN1157
CAS No.:1859-87-6
- H-Cys-OMe.HCl
Catalog No.:BCC2905
CAS No.:18598-63-5
- H-Ile-OMe.HCl
Catalog No.:BCC2964
CAS No.:18598-74-8
- AMD 3465 hexahydrobromide
Catalog No.:BCC5182
CAS No.:185991-07-5
- AMD 3465
Catalog No.:BCC5181
CAS No.:185991-24-6
- RS 79948 hydrochloride
Catalog No.:BCC6876
CAS No.:186002-54-0
- Kakuol
Catalog No.:BCN6455
CAS No.:18607-90-4
- Deacetylnimbin
Catalog No.:BCN4684
CAS No.:18609-16-0
- Quercetin-3-O-sophoroside
Catalog No.:BCN2771
CAS No.:18609-17-1
- 11alpha,12alpha-Epoxy-3beta,23-dihydroxy-30-norolean-20(29)-en-28,13beta-olide
Catalog No.:BCN1515
CAS No.:186140-36-3
- (-)-Anonaine
Catalog No.:BCN8235
CAS No.:1862-41-5
Adsorption mechanism of 2,4-dichlorophenoxyacetic acid onto nitric-acid-modified activated carbon fiber.[Pubmed:28379070]
Environ Technol. 2018 Apr;39(7):895-906.
Adsorption by carbon materials is one of the relatively fast methods in present research, which is widely used in emergency events. Activated carbon fiber (ACF) modified by nitric acid (N-ACF) was studied in this research to determine the adsorption performance for 2,4-dichlorophenoxyacetic acid (2,4-D). Subsequently, influence factors, adsorption isotherm models, kinetics and thermodynamic were investigated in a batch system to realize this adsorption. Experimental results showed that ACF modified by 0.1M nitric acid had a better removal ability than 2,4-D. Removal rate of 2,4-D by N-ACF was greatly influenced by pH with the optimum pH at 2. The superiority of the Langmuir isotherm model in describing the adsorption equilibrium was revealed by correlation coefficients R2 (R2 >/= 0.997). Furthermore, adsorption kinetics was well described by pseudo-second-order model. The results of thermodynamic showed that adsorption was a spontaneous, endothermic process with randomness increasing. Additionally, surface structure properties of adsorbent were characterized by Scanning electron microscopy, Fourier transform infrared spectroscopy, Specific surface area analysis of Brunauer, Emmett and Teller and Boehm's titration. It turned out that the micropore structure and functional groups on N-ACF all can contribute to the removal of 2,4-D.
Sorafenib and 2,3,5-triiodobenzoic acid-loaded imageable microspheres for transarterial embolization of a liver tumor.[Pubmed:28373713]
Sci Rep. 2017 Apr 3;7(1):554.
Sorafenib (SOF; an angiogenesis inhibitor) and 2,3,5-triiodobenzoic acid (TIBA; a contrast agent for computed tomography imaging)-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres (MSs) were fabricated. Embolization, drug delivery, and tracing the distribution of MSs for liver cancer therapy were accomplished with the developed MSs after their intra-arterial (IA) administration. SOF/TIBA/PLGA MSs with 24.8-28.5 microm mean diameters were prepared, and the sustained release of SOF from MSs was observed. Lower systemic exposure (represented as the area under the curve [AUC]) and maximum drug concentration in plasma (Cmax) values of the SOF/TIBA/PLGA MSs group (IA administration, 1 mg/kg) in the results of the pharmacokinetic study imply alleviated unwanted systemic effects (e.g., hand and foot syndrome), compared to the SOF solution group (oral administration, 10 mg/kg). In a rat hepatoma model, the increase of microvessel density (MVD) following arterial embolization (i.e., reactive angiogenesis) was partially limited by SOF/TIBA/PLGA MSs. This resulted in the SOF/TIBA/PLGA MSs group (IA administration, single dosing, 1 mg/kg) showing a smaller tumor size increase and viable tumor portion compared to the TIBA/PLGA MSs group. These findings suggest that a developed SOF/TIBA/PLGA MS can be a promising therapeutic system for liver cancer using a transarterial embolization strategy.
Automated synthesis of N-(2-[(18) F]Fluoropropionyl)-l-glutamic acid as an amino acid tracer for tumor imaging on a modified [(18) F]FDG synthesis module.[Pubmed:28370543]
J Labelled Comp Radiopharm. 2017 Jun 15;60(7):331-336.
N-(2-[(18) F]Fluoropropionyl)-l-glutamic acid ([(18) F]FPGLU) is a potential amino acid tracer for tumor imaging with positron emission tomography. However, due to the complicated multistep synthesis, the routine production of [(18) F]FPGLU presents many challenging laboratory requirements. To simplify the synthesis process of this interesting radiopharmaceutical, an efficient automated synthesis of [(18) F]FPGLU was performed on a modified commercial fluorodeoxyglucose synthesizer via a 2-step on-column hydrolysis procedure, including (18) F-fluorination and on-column hydrolysis reaction. [(18) F]FPGLU was synthesized in 12 +/- 2% (n = 10, uncorrected) radiochemical yield based on [(18) F]fluoride using the tosylated precursor 2. The radiochemical purity was >/=98%, and the overall synthesis time was 35 minutes. To further optimize the radiosynthesis conditions of [(18) F]FPGLU, a brominated precursor 3 was also used for the preparation of [(18) F]FPGLU, and the improved radiochemical yield was up to 20 +/- 3% (n = 10, uncorrected) in 35 minutes. Moreover, all these results were achieved using the similar on-column hydrolysis procedure on the modified fluorodeoxyglucose synthesis module.
2-Naphthoic acid ergosterol ester, an ergosterol derivative, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis.[Pubmed:28377207]
Steroids. 2017 Jun;122:9-15.
Phytosterol is a natural component of vegetable oil and includes ergosterol (ER) and beta-sitosterol. In this study, three new ergosterol monoester derivatives were obtained from the reflux reaction with ergosterol, organic acids (furoic acid, salicylic acid, and 2-naphthoic acid), EDCI, and DMAP in dichloromethane. The chemical structures were defined by IR and NMR. On the basis of the results, 2-naphthoic acid ergosterol ester (NE) had the highest tumor inhibition rate and was selected to study anti-tumor activity and its mechanism at doses of 0.025mmol/kg and 0.1mmol/kg in H22-tumor bearing mice. Compared with ER, NE exhibited more stronger anti-tumor activity in vivo. Furthermore, biochemical parameters of ALT, AST, BUN, and CRE showed that NE had little toxicity to mice. NE significantly improved serum cytokine levels of IFN-gamma and decreased VEGF levels. Moreover, H&E staining, TUNEL assay, immunohistochemistry, and western blotting indicated that NE exhibited anti-tumor activity in vivo by promoting apoptosis and inhibiting angiogenesis. In brief, the present study provided a method to improve ER anti-tumor activity and a reference for a new anti-tumor agent.


