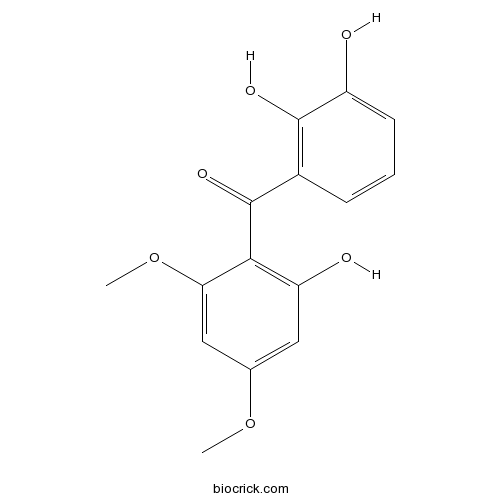
2,2',3'-Trihydroxy-4,6-dimethoxybenzophenoneCAS# 219861-73-1 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 219861-73-1 | SDF | Download SDF |
| PubChem ID | 10017078 | Appearance | Yellow powder |
| Formula | C15H14O6 | M.Wt | 290.3 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2,3-dihydroxyphenyl)-(2-hydroxy-4,6-dimethoxyphenyl)methanone | ||
| SMILES | COC1=CC(=C(C(=C1)OC)C(=O)C2=C(C(=CC=C2)O)O)O | ||
| Standard InChIKey | IYKKVJSOAPKTPD-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone Dilution Calculator

2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4447 mL | 17.2236 mL | 34.4471 mL | 68.8942 mL | 86.1178 mL |
| 5 mM | 0.6889 mL | 3.4447 mL | 6.8894 mL | 13.7788 mL | 17.2236 mL |
| 10 mM | 0.3445 mL | 1.7224 mL | 3.4447 mL | 6.8894 mL | 8.6118 mL |
| 50 mM | 0.0689 mL | 0.3445 mL | 0.6889 mL | 1.3779 mL | 1.7224 mL |
| 100 mM | 0.0344 mL | 0.1722 mL | 0.3445 mL | 0.6889 mL | 0.8612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Merresectine B
Catalog No.:BCN1918
CAS No.:219829-75-1
- Consiculine
Catalog No.:BCN1903
CAS No.:219829-73-9
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Bombiprenone
Catalog No.:BCN4940
CAS No.:21978-49-4
- ANA 12
Catalog No.:BCC6287
CAS No.:219766-25-3
- Taxezopidine L
Catalog No.:BCN6946
CAS No.:219749-76-5
- 14-Deoxy-12-hydroxyandrographolide
Catalog No.:BCN4673
CAS No.:219721-33-2
- Baicalin
Catalog No.:BCN5901
CAS No.:21967-41-9
- Griffipavixanthone
Catalog No.:BCN4939
CAS No.:219649-95-3
- (R)-Coclaurine
Catalog No.:BCN8348
CAS No.:2196-60-3
- beta-Hydroxypropiovanillone
Catalog No.:BCN4938
CAS No.:2196-18-1
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
9-(2'-Deoxy-2'-Fluoro-beta-d-Arabinofuranosyl) Adenine Is a Potent Antitrypanosomal Adenosine Analogue That Circumvents Transport-Related Drug Resistance.[Pubmed:28373184]
Antimicrob Agents Chemother. 2017 May 24;61(6). pii: AAC.02719-16.
Current chemotherapy against African sleeping sickness, a disease caused by the protozoan parasite Trypanosoma brucei, is limited by toxicity, inefficacy, and drug resistance. Nucleoside analogues have been successfully used to cure T. brucei-infected mice, but they have the limitation of mainly being taken up by the P2 nucleoside transporter, which, when mutated, is a common cause of multidrug resistance in T. brucei We report here that adenine arabinoside (Ara-A) and the newly tested drug 9-(2'-deoxy-2'-fluoro-beta-d-arabinofuranosyl) adenine (FANA-A) are instead taken up by the P1 nucleoside transporter, which is not associated with drug resistance. Like Ara-A, FANA-A was found to be resistant to cleavage by methylthioadenosine phosphorylase, an enzyme that protects T. brucei against the antitrypanosomal effects of deoxyadenosine. Another important factor behind the selectivity of nucleoside analogues is how well they are phosphorylated within the cell. We found that the T. brucei adenosine kinase had a higher catalytic efficiency with FANA-A than the mammalian enzyme, and T. brucei cells treated with FANA-A accumulated high levels of FANA-A triphosphate, which even surpassed the level of ATP and led to cell cycle arrest, inhibition of DNA synthesis, and the accumulation of DNA breaks. FANA-A inhibited nucleic acid biosynthesis and parasite proliferation with 50% effective concentrations (EC50s) in the low nanomolar range, whereas mammalian cell proliferation was inhibited in the micromolar range. Both Ara-A and FANA-A, in combination with deoxycoformycin, cured T. brucei-infected mice, but FANA-A did so at a dose 100 times lower than that of Ara-A.
Reprofiling of full-length phosphonated carbocyclic 2'-oxa-3'-aza-nucleosides toward antiproliferative agents: Synthesis, antiproliferative activity, and molecular docking study.[Pubmed:28371417]
Chem Biol Drug Des. 2017 Nov;90(5):679-689.
A series of phosphonated carbocyclic 2'-oxa-3'-aza-nucleosides were synthesized via 1,3 dipolar cycloaddition and evaluated for their in vitro antiproliferative activity against the growth of cancer cell lines (MCF-7, A2780, HCT116) and normal non-transformed fibroblast (MRC5) using MTT assay. Synthesized compounds exhibited antiproliferative activity in the micromolar range. Compounds 11b showed the highest activity against MCF-7 cells (IC50 of 0.2344 mum). Cell cycle analysis was performed for compound 11b on MCF7 cells showing arrest of cells in the S phase. Molecular docking of synthesized compounds confirmed high affinity of these compounds to two different receptors for anticancer nucleosides on dCK, namely the 1P5Z and 2ZIA, showing scores higher than the cognate ligand for all tested compounds. All synthesized compounds were evaluated according to the Lipinski, Veber, and Opera rules, and all of them passed the evaluation showing excellent features, superior to reference drugs. In addition, ADME for all the synthesized compounds was predicted through a theoretical kinetic study using the discovery studio 3.1 software.
The 2'-O-methyladenosine nucleoside modification gene OsTRM13 positively regulates salt stress tolerance in rice.[Pubmed:28369540]
J Exp Bot. 2017 Mar 1;68(7):1479-1491.
Stress induces changes of modified nucleosides in tRNA, and these changes can influence codon-anticodon interaction and therefore the translation of target proteins. Certain nucleoside modification genes are associated with regulation of stress tolerance and immune response in plants. In this study, we found a dramatic increase of 2'-O-methyladenosine (Am) nucleoside in rice seedlings subjected to salt stress and abscisic acid (ABA) treatment. We identified LOC_Os03g61750 (OsTRM13) as a rice candidate methyltransferase for the Am modification. OsTRM13 transcript levels increased significantly upon salt stress and ABA treatment, and the OsTrm13 protein was found to be located primarily to the nucleus. More importantly, OsTRM13 overexpression plants displayed improved salt stress tolerance, and vice versa, OsTRM13 RNA interference (RNAi) plants showed reduced tolerance. Furthermore, OsTRM13 complemented a yeast trm13Delta mutant, deficient in Am synthesis, and the purified OsTrm13 protein catalysed Am nucleoside formation on tRNA-Gly-GCC in vitro. Our results show that OsTRM13, encoding a rice tRNA nucleoside methyltransferase, is an important regulator of salt stress tolerance in rice.
Why does beta-cyclodextrin prefer to bind nucleotides with an adenine base rather than other 2'-deoxyribonucleoside 5'-monophosphates?[Pubmed:28365823]
J Mol Model. 2017 Apr;23(4):149.
beta-Cyclodextrin (beta-CD), which resides in the alpha-hemolysin (alphaHL) protein pore, can act as a molecular adapter in single-molecule exonuclease DNA sequencing approaches, where the different nucleotide binding behavior of beta-CD is crucial for base discrimination. In the present contribution, the inclusion modes of beta-CD towards four 2'-deoxyribonucleoside 5'-monophosphates (dNMPs) were investigated using quantum mechanics (QM) calculations. The calculated binding energy suggests that the binding affinity of dAMP to beta-CD are highest among all the dNMPs in solution, in agreement with experimental results. Geometry analysis shows that beta-CD in the dAMP complex undergoes a small conformational change, and weak interaction analysis indicates that there are small steric repulsion regions in beta-CD. These results suggest that beta-CD has lower geometric deformation energy in complexation with dAMP. Furthermore, topological analysis and weak interaction analysis suggest that the number and strength of intermolecular hydrogen bonds and van der Waals interactions are critical to dAMP binding, and they both make favorable contributions to the lower interaction energy. This work reveals the reason why beta-CD prefers to bind dAMP rather than other dNMPs, while opening exciting perspectives for the design of novel beta-CD-based molecular adapters in the single-molecule exonuclease method of sequencing DNA. Graphical Abstract The binding affinity of beta-cyclodextrin towards four 2'-deoxyribonucleoside 5'-monophosphates was investigated using quantum mechanics calculations.


