16-Epinormacusine BCAS# 126640-98-0 |
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- Tombozine
Catalog No.:BCN4117
CAS No.:604-99-9
- Koumidine
Catalog No.:BCX0689
CAS No.:1358-75-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126640-98-0 | SDF | Download SDF |
| PubChem ID | 137345987 | Appearance | Powder |
| Formula | C19H22N2O | M.Wt | 294.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,12R,13R,15E)-15-ethylidene-3,17-diazapentacyclo[12.3.1.02,10.04,9.012,17]octadeca-2(10),4,6,8-tetraen-13-yl]methanol | ||
| SMILES | CC=C1CN2C3CC1C(C2CC4=C3NC5=CC=CC=C45)CO | ||
| Standard InChIKey | VXTDUGOBAOLMED-GJCJBNIZSA-N | ||
| Standard InChI | InChI=1S/C19H22N2O/c1-2-11-9-21-17-8-14-12-5-3-4-6-16(12)20-19(14)18(21)7-13(11)15(17)10-22/h2-6,13,15,17-18,20,22H,7-10H2,1H3/b11-2-/t13?,15-,17-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
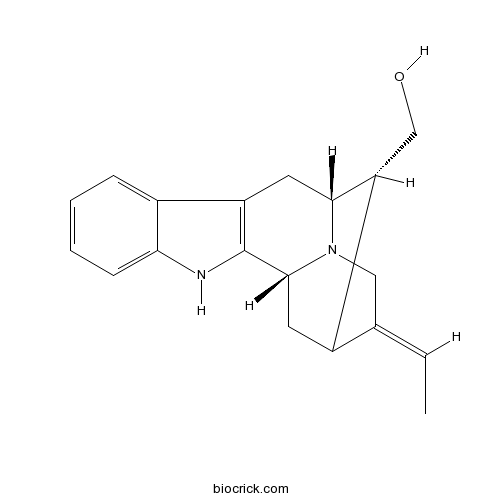
| Description | 16-Epinormacusine B is a natural product from Voacanga africana. |
| Structure Identification | Phytochemistry, 1991 , 30 (11) :3785-3792.Alkaloids from leaves and root bark ofErvatamia hirta.[Reference: WebLink]
|

16-Epinormacusine B Dilution Calculator

16-Epinormacusine B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3967 mL | 16.9837 mL | 33.9674 mL | 67.9348 mL | 84.9185 mL |
| 5 mM | 0.6793 mL | 3.3967 mL | 6.7935 mL | 13.587 mL | 16.9837 mL |
| 10 mM | 0.3397 mL | 1.6984 mL | 3.3967 mL | 6.7935 mL | 8.4918 mL |
| 50 mM | 0.0679 mL | 0.3397 mL | 0.6793 mL | 1.3587 mL | 1.6984 mL |
| 100 mM | 0.034 mL | 0.1698 mL | 0.3397 mL | 0.6793 mL | 0.8492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A 887826
Catalog No.:BCC7898
CAS No.:1266212-81-0
- Trilobinine
Catalog No.:BCN7927
CAS No.:126595-92-4
- Vallesamine N-oxide
Catalog No.:BCN6144
CAS No.:126594-73-8
- Cyclocerberidol
Catalog No.:BCN6143
CAS No.:126594-66-9
- Cerberidol
Catalog No.:BCN6142
CAS No.:126594-64-7
- S1RA hydrochloride
Catalog No.:BCC4190
CAS No.:1265917-14-3
- 8,14-Epoxyergosta-4,22-diene-3,6-dione
Catalog No.:BCN1591
CAS No.:1265908-20-0
- Ciclesonide
Catalog No.:BCC5234
CAS No.:126544-47-6
- CH5183284 (Debio-1347)
Catalog No.:BCC5649
CAS No.:1265229-25-1
- Mupirocin
Catalog No.:BCC5558
CAS No.:12650-69-0
- Metasequirin D
Catalog No.:BCN4781
CAS No.:1264694-96-3
- Dihydroeponemycin
Catalog No.:BCC3596
CAS No.:126463-64-7
- KT 5823
Catalog No.:BCC7006
CAS No.:126643-37-6
- UTPγS trisodium salt
Catalog No.:BCC7624
CAS No.:1266569-94-1
- Isoaltenuene
Catalog No.:BCN7313
CAS No.:126671-80-5
- Gancaonin G
Catalog No.:BCN6837
CAS No.:126716-34-5
- Gancaonin I
Catalog No.:BCN7144
CAS No.:126716-36-7
- Tilifodiolide
Catalog No.:BCN6145
CAS No.:126724-95-6
- Acetylsventenic acid
Catalog No.:BCN4849
CAS No.:126737-42-6
- Sarafotoxin S6a
Catalog No.:BCC5834
CAS No.:126738-34-9
- GR 89696 fumarate
Catalog No.:BCC7083
CAS No.:126766-32-3
- Sventenic acid
Catalog No.:BCN3923
CAS No.:126778-79-8
- Ulipristal acetate
Catalog No.:BCC4068
CAS No.:126784-99-4
- 5,7,3'-Trihydroxy-4'-methoxy-8-prenylflavanone
Catalog No.:BCN1590
CAS No.:1268140-15-3
Enantiospecific total synthesis of (-)-(E)16-epiaffinisine, (+)-(E)16-epinormacusine B, and (+)-dehydro-16-epiaffinisine as well as the stereocontrolled total synthesis of alkaloid G.[Pubmed:12868917]
J Org Chem. 2003 Jul 25;68(15):5852-9.
An efficient strategy is described for the total synthesis of the sarpagine-related indole alkaloids (-)-(E)16-epiaffinisine (1), (+)-(E)16-Epinormacusine B (2), and (+)-dehydro-16-epiaffinisine (4). A key step employed the chemospecific and regiospecific hydroboration/oxidation at C(16)-C(17); this method has also resulted in the synthesis of (+)-dehydro-16-Epinormacusine B (5). The oxy-anion Cope rearrangement followed by protonation of the enolate that resulted under conditions of kinetic control has been employed to generate the key asymmetric centers at C(15), C(16), and C(20) in alkaloid G (7) in a highly stereocontrolled fashion (>43:1). Conditions that favor control of the sarpagine stereochemistry at C(16) vs the epimeric ajmaline configuration at the same stereocenter have been determined. The formation of the required cyclic ether in 4, 5, and 7 was realized with complete control from the top face on treatment of the corresponding alcohols with DDQ/THF or DDQ/aq THF in excellent yields.
Stereospecific, enantiospecific total synthesis of the sarpagine indole alkaloids (E)16-epiaffinisine, (E)16-epinormacusine B, and dehydro-16-epiaffinisine.[Pubmed:12489960]
Org Lett. 2002 Dec 26;4(26):4681-4.
[reaction: see text] The first stereospecific total synthesis of the sarpagine indole alkaloids (E)16-epiaffinisine (1), (E)16-Epinormacusine B (2), and dehydro-16-epiaffinisine (4) has been completed; this method has also resulted in the synthesis of dehydro-16-Epinormacusine B (5). The formation of the required ether in both 4 and 5 was realized with complete control from the top face on treatment of the corresponding alcohols with DDQ/THF in 98% and 95% yields, respectively.


