12-HydroxymyricanoneCAS# 191999-68-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 191999-68-5 | SDF | Download SDF |
| PubChem ID | 10714326 | Appearance | Powder |
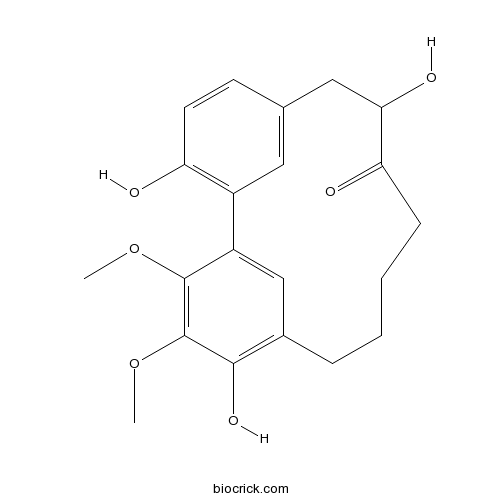
| Formula | C21H24O6 | M.Wt | 372.41 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | COC1=C(C(=C2CCCCC(=O)C(CC3=CC(=C(C=C3)O)C1=C2)O)O)OC | ||
| Standard InChIKey | MZTZAESUYFUQBV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H24O6/c1-26-20-15-11-13(19(25)21(20)27-2)5-3-4-6-17(23)18(24)10-12-7-8-16(22)14(15)9-12/h7-9,11,18,22,24-25H,3-6,10H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 12-Hydroxymyricanone exhibits anti-tubercular activity against Mycobacterium tuberculosis H37Rv in vitro and the MIC value of 35.8 ug/mL. 2. 12-Hydroxymyricanone inhibits the release of nitric oxide with the IC(50) value of 30.19 muM. |
| Targets | Antifection | NO | NOS |

12-Hydroxymyricanone Dilution Calculator

12-Hydroxymyricanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6852 mL | 13.4261 mL | 26.8521 mL | 53.7043 mL | 67.1303 mL |
| 5 mM | 0.537 mL | 2.6852 mL | 5.3704 mL | 10.7409 mL | 13.4261 mL |
| 10 mM | 0.2685 mL | 1.3426 mL | 2.6852 mL | 5.3704 mL | 6.713 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.537 mL | 1.0741 mL | 1.3426 mL |
| 100 mM | 0.0269 mL | 0.1343 mL | 0.2685 mL | 0.537 mL | 0.6713 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- HIV-1 Tat Protein Peptide
Catalog No.:BCC4417
CAS No.:191936-91-1
- NTNCB hydrochloride
Catalog No.:BCC7270
CAS No.:191931-56-3
- Flutax 1
Catalog No.:BCC7298
CAS No.:191930-58-2
- BIBO 3304 trifluoroacetate
Catalog No.:BCC7355
CAS No.:191868-14-1
- Deoxypodophyllotoxin
Catalog No.:BCN1182
CAS No.:19186-35-7
- Cyclo(Pro-Gly)
Catalog No.:BCN2417
CAS No.:19179-12-5
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Tenacissoside H
Catalog No.:BCN2570
CAS No.:191729-45-0
- Tenacissoside I
Catalog No.:BCN4681
CAS No.:191729-44-9
- Tenacissoside G
Catalog No.:BCN4682
CAS No.:191729-43-8
- Pomalidomide (CC-4047)
Catalog No.:BCC2246
CAS No.:19171-19-8
- SIB 1553A hydrochloride
Catalog No.:BCC6284
CAS No.:191611-89-9
- Hinokiflavone
Catalog No.:BCN2989
CAS No.:19202-36-9
- Tenulin
Catalog No.:BCN7961
CAS No.:19202-92-7
- MRS 1334
Catalog No.:BCC5753
CAS No.:192053-05-7
- Harpagoside
Catalog No.:BCN4995
CAS No.:19210-12-9
- Prazosin
Catalog No.:BCC4081
CAS No.:19216-56-9
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- Galanganone A
Catalog No.:BCN7484
CAS No.:1922129-42-7
- Galanganone B
Catalog No.:BCN7485
CAS No.:1922129-43-8
- Galanganone C
Catalog No.:BCN7486
CAS No.:1922129-46-1
- SIB 1508Y maleate
Catalog No.:BCC7975
CAS No.:192231-16-6
- CGP 71683 hydrochloride
Catalog No.:BCC7283
CAS No.:192322-50-2
Biological evaluation of secondary metabolites from the roots of Myrica adenophora.[Pubmed:24810013]
Phytochemistry. 2014 Jul;103:89-98.
Bioassay-guided fractionation of the roots of Myrica adenophora led to isolation of 24 known compounds and hitherto unknown compounds, including three A-type proanthocyanidins [adenodimerins A-C], two esters of sucrose [myricadenins A and B ], and the phenolic glycoside 6'-O-galloyl orbicularin. Spectroscopic analyses were used to determine their structures. Adenodimerin A, myricananin C, and myricetin showed strong 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities, with SC50 values of 7.9, 16.3, and 15.9 muM, respectively. Adenodimerin A, myricanone, myricananin C, (-)-myricanol, myricanol 11-O-beta-D-glucopyranoside, and myricetin showed stronger 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging activities than the positive control, with SC50 values of 7.5, 19.6, 12.0, 22.3, 19.6, and 15.6 muM, respectively. 5-Deoxymyricanone, porson, 12-Hydroxymyricanone (-)-myricanol, and (+)-galeon exhibited anti-tubercular activity against Mycobacterium tuberculosis H37Rv in vitro and MICs values of 25.8, 40.0, 35.8, 30.0, and 15.0 mug/mL, respectively. Myricadenin A, myricanone, myricananin C, and (-)-myricanol exhibited anti-inflammatory activities in the iNOS assay with EC50 values of 18.1, 1.00, 13.0, and 7.5 muM, respectively.
Cyclic diarylheptanoids from Myrica nana inhibiting nitric oxide release.[Pubmed:18723353]
Bioorg Med Chem. 2008 Sep 15;16(18):8510-5.
Investigation of the roots of Myrica nana afforded five new cyclic diarylheptanoids, myricananins A-E (1-5), two new artifacts of myricananins A and B (6-7), and four known compounds, 12-Hydroxymyricanone (8), alnusonol (9), myricatomentogenin (10), and actinidione (11). The structures of these new compounds were established by detailed spectroscopic methods. The stereochemistry of compounds 1 and 2 were determined by single-crystal X-ray diffraction. In exception of compounds 2, 6 and 10, all the other compounds were examined for their inhibitory effects on nitric oxide production in lipopolysaccharides-activated macrophages. Compounds 1, 3, 7, 8 and 9 inhibited the release of nitric oxide with IC(50) values of 45.32, 63.51, 52.81, 30.19 and 46.18muM, respectively. Furthermore, compound 1 was found to inhibit the expression of inducible nitric oxide synthase.


