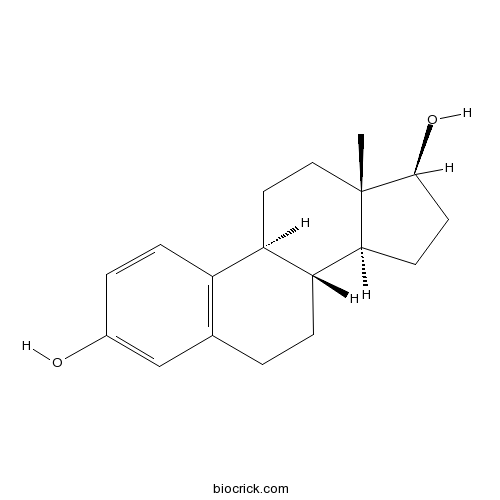
beta-EstradiolSex hormone CAS# 50-28-2 |
- Catalpol
Catalog No.:BCN5094
CAS No.:2415-24-9
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Ethinyl Estradiol
Catalog No.:BCC3777
CAS No.:57-63-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 50-28-2 | SDF | Download SDF |
| PubChem ID | 5757 | Appearance | Powder |
| Formula | C18H24O2 | M.Wt | 272.38 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Synonyms | 17 β-Estradiol | ||
| Solubility | DMSO : ≥ 50 mg/mL (183.57 mM) Ethanol : 20 mg/mL (73.43 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol | ||
| SMILES | CC12CCC3C(C1CCC2O)CCC4=C3C=CC(=C4)O | ||
| Standard InChIKey | VOXZDWNPVJITMN-ZBRFXRBCSA-N | ||
| Standard InChI | InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Estradiol, or more precisely, 17β-estradiol, is a human sex hormone and steroid, and the primary female sex hormone. Beta-estradiol has been considered to be a neurotrophic agent, beta-estradiol at a dose of 0.25 microg/day prevents ischemia-induced learning disability and neuronal loss at early stages after transient forebrain ischemia, possibly via a receptor-mediated pathway without attenuating free radical neurotoxicity. |
| Targets | LDL |
| In vitro | β-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells.[Pubmed: 25913303]FEBS J. 2015 Jul;282(14):2682-96.The lower risk of coronary artery disease in premenopausal women than in men and postmenopausal women implicates sex steroids in cardioprotective processes. beta-Estradiol upregulates liver low-density lipoprotein receptor (LDLR), which, in turn, decreases circulating levels of low-density lipoprotein, which is a risk factor for coronary artery disease. Conversely, LDLR protein is negatively regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9).
|
| In vivo | Gonadal status-dependent effects of in vivo β-estradiol administration to female rats on in vitro epileptiform activity induced by low [Mg2+]₀ in combined hippocampus-entorhinal cortex slices.[Pubmed: 24113171]Epilepsy Res. 2013 Dec;107(3):297-301.There are controversial data regarding estrogen effects on neuronal excitability.
|
| Animal Research | Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil.[Pubmed: 9527626]Neurosci Res. 1997 Dec;29(4):345-54.beta-Estradiol has been considered to be a neurotrophic agent, but its in vivo effect on gerbils with transient forebrain ischemia has not yet been demonstrated.
|

beta-Estradiol Dilution Calculator

beta-Estradiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6713 mL | 18.3567 mL | 36.7134 mL | 73.4268 mL | 91.7835 mL |
| 5 mM | 0.7343 mL | 3.6713 mL | 7.3427 mL | 14.6854 mL | 18.3567 mL |
| 10 mM | 0.3671 mL | 1.8357 mL | 3.6713 mL | 7.3427 mL | 9.1784 mL |
| 50 mM | 0.0734 mL | 0.3671 mL | 0.7343 mL | 1.4685 mL | 1.8357 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3671 mL | 0.7343 mL | 0.9178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Estradiol is the main sex hormone present in females and also present in males as an active metabolic product of testosterone. It is the major estrogen which impacts on reproductive and organs such as the bones in humans [1].
As a main form of estrogen, estradiol interacts with two estrogen receptors (ER), i.e ERα and ERβ, which exert the effects by diverse signaling pathways that mediate the nongenomic and genomic cases.
With treatment of estradiol, ERβ-specific effects on gene expression have been investigated in three different cell lines lacking expression of endogenous ERα and ERβ, namely U2OS (25), HEK293 (26), and Hs578T (23) cells. 17 genes of the 76 ERβ-regulated genes were commonly regulated by both ERα and ERβ, suggesting that the transcriptional effects of estradiol via ERα or ERβ are largely distinct in U2OS cells. 95 and 61 genes were identified as ERβ-regulated genes in Hs578T cells and HEK293 cells in a 24-h estradiol treatment, respectively. Only three genes (PTGER4, ENPP2, and DKK1) commonly regulated in both HEK293 and Hs578T cells, suggesting that ERβ evokes distinct gene responses in different types of target cells. By using estradiol, new roles of ERβ signaling was established, including protective functions in the epithelial-mesenchymal transition,as well as regulation of cell proliferation in the colon [2].
Animal experiments have shown the protective effects of estrogens in cardiovascular diseases. In mice and in vitro in human aorta endothelial cells, treatment with estradiol increases the expression of mitochondrial superoxide dismutase significantly. Estradiol up-regulates superoxide dismutase by tethering of estrogen receptor to Sp1 and the increased binding of Sp1 to GC-box on the superoxide dismutase promoter, where ERα responses estradiol-mediated activation of gene, and ERβ maintains a level of basal gene expression. Investigation of the reduction in PS levels caused by E2 in HepG2-ERα cells showed E2 repressed the production of mRNA and antigen of PS. In addition, E2 might repress PROS1 transcription depending upon ERα-Sp1 recruiting transcriptional repressors in HepG2-ERα cells and, the high levels of E2 resulting in reduced levels of plasma PS would be a risk for deep venous thrombosis in pregnant women [1,3].
References:
[1]. Liu ZY, Gou YL, Zhang HY, et al. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biology, 2014, 3: 88-99.
[2]. Zhao CY, Dahlman-Wright K, Gustafsson J. Estrogen signaling via estrogen
receptor β*. The Journal Of Biological Chemistry, 2010, 51: 39575–39579.
[3]. Suzuki A, Sanda N, Miyawaki Y, et al. Down-regulation of pros1 gene expression by 17 beta-estradiol via estrogen receptor alpha (er alpha)-sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-hdac3 complex. Journal Of Biological Chemistry, 2010, 285(18): 13444-13453.
- Estriol
Catalog No.:BCN2235
CAS No.:50-27-1
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Corticosterone
Catalog No.:BCN2203
CAS No.:50-22-6
- Cyclophosphamide
Catalog No.:BCC1185
CAS No.:50-18-0
- Ergocalciferol
Catalog No.:BCN2208
CAS No.:50-14-6
- Mitomycin C
Catalog No.:BCC2388
CAS No.:50-07-7
- Cortisone acetate
Catalog No.:BCC4771
CAS No.:50-04-4
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Guanidine HCl
Catalog No.:BCC4785
CAS No.:50-01-1
- Tioxolone
Catalog No.:BCC2316
CAS No.:4991-65-5
- 2,4-Pyridinedicarboxylic Acid
Catalog No.:BCC6483
CAS No.:499-80-9
- Phenylbutazone
Catalog No.:BCC4822
CAS No.:50-33-9
- Thalidomide
Catalog No.:BCC2248
CAS No.:50-35-1
- Cocaine
Catalog No.:BCN1901
CAS No.:50-36-2
- Clomiphene citrate
Catalog No.:BCC4480
CAS No.:50-41-9
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Estradiol Benzoate
Catalog No.:BCC4779
CAS No.:50-50-0
- Reserpine
Catalog No.:BCN4960
CAS No.:50-55-5
- Oxytocin
Catalog No.:BCC5435
CAS No.:50-56-6
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
Gonadal status-dependent effects of in vivo beta-estradiol administration to female rats on in vitro epileptiform activity induced by low [Mg2+](0) in combined hippocampus-entorhinal cortex slices.[Pubmed:24113171]
Epilepsy Res. 2013 Dec;107(3):297-301.
There are controversial data regarding estrogen effects on neuronal excitability. We investigated whether beta-Estradiol (EB) administration to ovariectomized (OVX) or gonadally intact female rats alters epileptiform activity within the dentate gyrus network induced in vitro by removing [Mg2+]o in combined hippocampus-entorhinal cortex slices. In vivo EB administration significantly influenced the epileptiform activity in gonadal status-dependent manner. The onset of epileptiform discharges was modestly delayed in slices from OVX rats replaced with physiologically relevant doses of EB but the number of discharges was not affected. In contrast, EB administration to gonadally intact rats had robust effects such that: EB delayed the onset of discharges but significantly increased their number within the dentate gyrus network. Our data suggest that EB in physiologically relevant concentrations does not seem to negatively affect hippocampal neuronal excitability, nevertheless supraphysiological EB levels may enhance seizure severity.
beta-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells.[Pubmed:25913303]
FEBS J. 2015 Jul;282(14):2682-96.
The lower risk of coronary artery disease in premenopausal women than in men and postmenopausal women implicates sex steroids in cardioprotective processes. beta-Estradiol upregulates liver low-density lipoprotein receptor (LDLR), which, in turn, decreases circulating levels of low-density lipoprotein, which is a risk factor for coronary artery disease. Conversely, LDLR protein is negatively regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9). Herein, we investigated PCSK9 regulation by beta-Estradiol and its impact on LDLR in human hepatocarcinoma HuH7 cells grown in the presence or absence of beta-Estradiol. Immunoblot analysis showed upregulation of LDLR at 3 mum beta-Estradiol (140%), and the upregulation reached 220% at 10 mum beta-Estradiol; only at the latter dose was an increase in LDLR mRNA detected by qPCR, suggesting post-translational regulation of LDLR. No changes in PCSK9 mRNA or secreted protein levels were detected by qPCR or ELISA, respectively. beta-Estradiol-conditioned medium devoid of PCSK9 failed to upregulate LDLR. Similarly, PCSK9 knockdown cells showed no upregulation of LDLR by beta-Estradiol. Together, these results indicate a requirement for PCSK9 in the beta-Estradiol-induced upregulation of LDLR. A radiolabeling assay showed a significant, dose-dependent decrease in the ratio of secreted phosphoPCSK9 to total secreted PCSK9 with increasing beta-Estradiol levels, suggesting a change in the functional state of PCSK9 in the presence of beta-Estradiol. Our results indicate that the protein upregulation of LDLR at subtranscriptionally effective doses of beta-Estradiol, and its supratranscriptional upregulation at 10 mum beta-Estradiol, occur through an extracellular PCSK9-dependent mechanism.
Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil.[Pubmed:9527626]
Neurosci Res. 1997 Dec;29(4):345-54.
beta-Estradiol has been considered to be a neurotrophic agent, but its in vivo effect on gerbils with transient forebrain ischemia has not yet been demonstrated. In the first set of the present experiments, we infused beta-Estradiol at a dose of 0.05 or 0.25 microg/day for 7 days into the lateral ventricles of normothermic gerbils starting 2 h before 3-min forebrain ischemia. beta-Estradiol infusion at a dose of 0.25 microg/day prevented significantly the ischemia-induced reduction of response latency time as revealed by a step-down passive avoidance task. Subsequent light and electron microscopic examinations showed that pyramidal neurons in the hippocampal CA1 region as well as synapses within the strata moleculare, radiatum and oriens of the region were significantly more numerous in gerbils infused with beta-Estradiol than in those receiving saline infusion. beta-Estradiol at a dose of 1.25 microg/day was ineffective and occasionally increased the mortality of experimental animals. Since the total brain content of exogenous beta-Estradiol at 12 h after forebrain ischemia was estimated to be less than 145 ng, the second set of experiments focused on the neurotrophic action of beta-Estradiol at concentrations around 100 ng/ml in vitro. beta-Estradiol at concentrations of 1-100 ng/ml facilitated the survival and process extension of cultured hippocampal neurons, but it did not exhibit any significant radical-scavenging effects at the concentration range. On the other hand, 100 microg/ml of beta-Estradiol, even though failing to support hippocampal neurons in vitro, effectively scavenged free radicals in subsequent in vitro studies, as demonstrated elsewhere. These findings suggest that beta-Estradiol at a dose of 0.25 microg/day prevents ischemia-induced learning disability and neuronal loss at early stages after transient forebrain ischemia, possibly via a receptor-mediated pathway without attenuating free radical neurotoxicity.
Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells.[Pubmed:15539556]
Endocrinology. 2005 Feb;146(2):624-32.
Although nonclassical estrogen actions initiated at the cell surface have been described in many tissues, the identities of the membrane estrogen receptors (mERs) mediating these actions remain unclear. Here we show that GPR30, an orphan receptor unrelated to nuclear estrogen receptors, has all the binding and signaling characteristics of a mER. A high-affinity (dissociation constant 2.7 nm), limited capacity, displaceable, single binding site specific for estrogens was detected in plasma membranes of SKBR3 breast cancer cells that express GPR30 but lack nuclear estrogen receptors. Progesterone-induced increases and small interfering RNA-induced decreases in GPR30 expression in SKBR3 cells were accompanied by parallel changes in specific estradiol-17beta (E2) binding. Plasma membranes of human embryonic kidney 293 cells transfected with GPR30, but not those of untransfected cells, and human placental tissues that express GPR30 also displayed high-affinity, specific estrogen binding typical of mERs. E2 treatment of transfected cell membranes caused activation of a stimulatory G protein that is directly coupled to the receptor, indicating GPR30 is a G protein-coupled receptor (GPCR), and also increased adenylyl cyclase activity. The finding that the antiestrogens tamoxifen and ICI 182,780, and an environmental estrogen, ortho,para-dichlorodiphenyldichloroethylene (o,p'-DDE), have high binding affinities to the receptor and mimic the actions of E2 has important implications for both the development and treatment of estrogen-dependent breast cancer. GPR30 is structurally unrelated to the recently discovered family of GPCR-like membrane progestin receptors. The identification of a second distinct class of GPCR-like steroid membrane receptors suggests a widespread role for GPCRs in nonclassical steroid hormone actions.
Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta.[Pubmed:9048584]
Endocrinology. 1997 Mar;138(3):863-70.
The rat estrogen receptor (ER) exists as two subtypes, ER alpha and ER beta, which differ in the C-terminal ligand binding domain and in the N-terminal transactivation domain. In this study we investigated the messenger RNA expression of both ER subtypes in rat tissues by RT-PCR and compared the ligand binding specificity of the ER subtypes. Saturation ligand binding analysis of in vitro synthesized human ER alpha and rat ER beta protein revealed a single binding component for 16 alpha-iodo-17 beta-Estradiol with high affinity [dissociation constant (Kd) = 0.1 nM for ER alpha protein and 0.4 nM for ER beta protein]. Most estrogenic substances or estrogenic antagonists compete with 16 alpha-[125I]iodo-17 beta-Estradiol for binding to both ER subtypes in a very similar preference and degree; that is, diethylstilbestrol > hexestrol > dienestrol > 4-OH-tamoxifen > 17 beta-Estradiol > coumestrol, ICI-164384 > estrone, 17 alpha-estradiol > nafoxidine, moxestrol > clomifene > estriol, 4-OH-estradiol > tamoxifen, 2-OH-estradiol, 5-androstene-3 beta, 17 beta-diol, genistein for the ER alpha protein and dienestrol > 4-OH-tamoxifen > diethylstilbestrol > hexestrol > coumestrol, ICI-164384 > 17 beta-Estradiol > estrone, genistein > estriol > nafoxidine, 5-androstene-3 beta, 17 beta-diol > 17 alpha-estradiol, clomifene, 2-OH-estradiol > 4-OH-estradiol, tamoxifen, moxestrol for the ER beta protein. The rat tissue distribution and/or the relative level of ER alpha and ER beta expression seems to be quite different, i.e. moderate to high expression in uterus, testis, pituitary, ovary, kidney, epididymis, and adrenal for ER alpha and prostate, ovary, lung, bladder, brain, uterus, and testis for ER beta. The described differences between the ER subtypes in relative ligand binding affinity and tissue distribution could contribute to the selective action of ER agonists and antagonists in different tissues.


