Vildagliptin (LAF-237)DPP-4 inhibitor CAS# 274901-16-5 |
- Granisetron HCl
Catalog No.:BCC1060
CAS No.:107007-99-8
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
Quality Control & MSDS
Number of papers citing our products

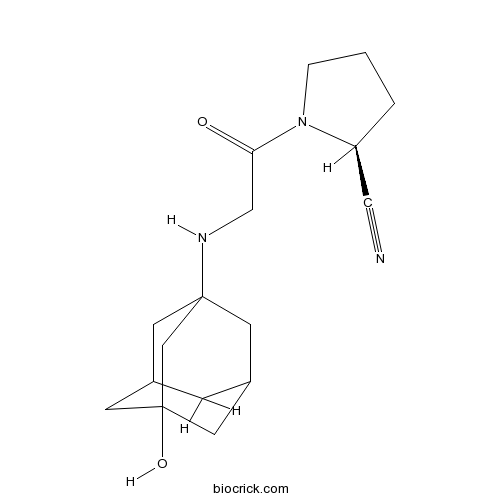
Chemical structure
3D structure
| Cas No. | 274901-16-5 | SDF | Download SDF |
| PubChem ID | 6918537 | Appearance | Powder |
| Formula | C17H25N3O2 | M.Wt | 303.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LAF237; NVP-LAF 237 | ||
| Solubility | H2O : 50 mg/mL (164.80 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-1-[2-[(3-hydroxy-1-adamantyl)amino]acetyl]pyrrolidine-2-carbonitrile | ||
| SMILES | C1CC(N(C1)C(=O)CNC23CC4CC(C2)CC(C4)(C3)O)C#N | ||
| Standard InChIKey | SYOKIDBDQMKNDQ-XWTIBIIYSA-N | ||
| Standard InChI | InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vildagliptin is a potent inhibitor of DDP-4 with IC50 value of 62 nM. | |||||
| Targets | DDP-4 | |||||
| IC50 | 62 nM | |||||

Vildagliptin (LAF-237) Dilution Calculator

Vildagliptin (LAF-237) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.296 mL | 16.4799 mL | 32.9598 mL | 65.9196 mL | 82.3995 mL |
| 5 mM | 0.6592 mL | 3.296 mL | 6.592 mL | 13.1839 mL | 16.4799 mL |
| 10 mM | 0.3296 mL | 1.648 mL | 3.296 mL | 6.592 mL | 8.2399 mL |
| 50 mM | 0.0659 mL | 0.3296 mL | 0.6592 mL | 1.3184 mL | 1.648 mL |
| 100 mM | 0.033 mL | 0.1648 mL | 0.3296 mL | 0.6592 mL | 0.824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LAF-237 is a potent inhibitor of DDP-4 with IC50 value of 2.3 nM [1].
DDP-4 is an antigenic enzyme expressed on the surface of most cell types and is associated with signal transduction, apoptosis and immune regulation [1].
LAF-237 is a specific inhibitor of DDP4 and can reduce glycemia. When tested on Type II diabetes patients, LAF-237 inhibits the inactivation of GLP-1 by DPP-4, allowing GLP-1 to increase the secretion of insulin and decrease glucagon release [2].
In the glucose tolerance test with obese male Zucker rats, LAF-237 can significantly decrease glucose excursion and stimulates insulin secretion. There are 95% inhibition activities of DDP-4 two hours after the injection of LAF-237[3]. Through enhances beta cell replication and reduces apoptosis, LAF-237 increases in the number of beta cell and can sustain for 12 days after washout LAF-237. Also, LAF-237 can protect nerve fiber loss in STZ-induced diabetic adult male Sprague Dawley rats [4].
References:
[1]. Chakraborty S, Rendón-Ramírez A, Ásgeirsson B, et al. The dipeptidyl peptidase IV inhibitors vildagliptin and K-579 inhibit a phospholipase C: a case of promiscuous scaffolds in proteins. F1000Res, 2013, 2: 286.
[2]. Ahrén B, Landin-Olsson M, Jansson PA, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism, 2004, 89 (5): 2078–84.
[3] Villhauer EB, et al. 1-[[(3-hydroxy-1-adamantyl) amino] acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem, 2003, 46(13), 2774-2789.
[4] Duttaroy A, et al. Combination therapy with nateglinide and vildagliptin improves postprandial metabolic derangements in Zucker fatty rats. Eur J Pharmacol, 2011, 650(2-3), 703-707.
- 2-O-Acetyltutin
Catalog No.:BCN5163
CAS No.:2749-28-2
- L-Ala-ol
Catalog No.:BCC2590
CAS No.:2749-11-3
- trans-4-Aminocyclohexanol
Catalog No.:BCC9181
CAS No.:27489-62-9
- Cyclo(D-Val-L-Pro)
Catalog No.:BCN4015
CAS No.:27483-18-7
- O-Benzyldauricine
Catalog No.:BCC8222
CAS No.:2748-99-4
- H-Leu-OtBu.HCl
Catalog No.:BCC2974
CAS No.:2748-02-9
- 7-Acetoxy-4-methylcoumarin
Catalog No.:BCC8775
CAS No.:2747-05-9
- LE 300
Catalog No.:BCC7148
CAS No.:274694-98-3
- Ticagrelor
Catalog No.:BCC4975
CAS No.:274693-27-5
- Neocryptotanshinone II
Catalog No.:BCN3138
CAS No.:27468-20-8
- Xanthohumol D
Catalog No.:BCN5162
CAS No.:274675-25-1
- 5-Methoxyisatin
Catalog No.:BCC8098
CAS No.:39755-95-8
- Protostemonine
Catalog No.:BCN8172
CAS No.:27495-40-5
- Physalin C
Catalog No.:BCN7918
CAS No.:27503-33-9
- O-Methylpallidine
Catalog No.:BCN3916
CAS No.:27510-33-4
- Mesaconitine
Catalog No.:BCN5987
CAS No.:2752-64-9
- Gambogic acid
Catalog No.:BCN2318
CAS No.:2752-65-0
- H-Cha-OH
Catalog No.:BCC2663
CAS No.:27527-05-5
- Gedunin
Catalog No.:BCC7676
CAS No.:2753-30-2
- Feretoside
Catalog No.:BCN5164
CAS No.:27530-67-2
- H-Gly-OtBu.HCl
Catalog No.:BCC2952
CAS No.:27532-96-3
- MS 245 oxalate
Catalog No.:BCC6127
CAS No.:275363-58-1
- Baccatin III
Catalog No.:BCN5165
CAS No.:27548-93-2
- MMF
Catalog No.:BCC7941
CAS No.:2756-87-8
Vildagliptin: the evidence for its place in the treatment of type 2 diabetes mellitus.[Pubmed:20694081]
Core Evid. 2008 Jun;3(1):13-30.
INTRODUCTION: Type 2 diabetes is increasing in prevalence worldwide and is a leading cause of morbidity and mortality, mainly due to the development of complications. Vildagliptin is an inhibitor of dipeptidyl peptidase 4 (DPP-4), a new class of oral antidiabetic agents. AIMS: To evaluate the role of vildagliptin in the management of type 2 diabetes. EVIDENCE REVIEW: Clear evidence shows that vildagliptin improves glycemic control (measured by glycosylated hemoglobin and blood glucose levels) more than placebo in adults with type 2 diabetes, either as monotherapy or in combination with metformin. Vildagliptin is as effective as pioglitazone and rosiglitazone, and slightly less effective than metformin, although better tolerated. Further glycemic control is achieved when adding vildagliptin to metformin, pioglitazone, or glimepride. There is evidence that vildagliptin improves beta-cell function and insulin sensitivity. Vildagliptin does not appear to be associated with weight gain or with a higher risk of hypoglycemia than placebo or other commonly used oral antidiabetic agents. Economic evidence is currently lacking. PLACE IN THERAPY: Vildagliptin improves glycemic control with little if any weight gain or hypoglycemia in adult patients with type 2 diabetes when given alone or in combination with metformin, thiazolidinediones, or sulfonylureas. Since many diabetic patients require combination therapy, the complementary mechanism of action of vildagliptin and other commonly prescribed antidiabetic drugs represents an important new therapeutic option in diabetes management.
DPP-4 inhibitors and their potential role in the management of type 2 diabetes.[Pubmed:17073841]
Int J Clin Pract. 2006 Nov;60(11):1454-70.
The dipeptidyl peptidase 4 (DPP-4) inhibitors enhance the body's own ability to control blood glucose by increasing the active levels of incretin hormones in the body. Their mechanism of action is distinct from any existing class of oral glucose-lowering agents. They control elevated blood glucose by triggering pancreatic insulin secretion, suppressing pancreatic glucagon secretion, and signalling the liver to reduce glucose production. The leading DPP-4 inhibitors have shown clinically significant HbA1c reductions up to 1 year of treatment and offer many potential advantages over existing diabetes therapies including a low risk of hypoglycaemia, no effect on body weight, and the potential, based on animal and in vitro studies, for the regeneration and differentiation of pancreatic beta-cells. They are efficacious as monotherapy and also in combination with commonly prescribed antidiabetic agents and are suitable for once-daily oral dosing. Consequently, many DPP-4 inhibitors such as vildagliptin (Galvus; LAF-237), sitagliptin (Januvia; MK-0431), and saxagliptin (BMS-477118) have advanced into late-stage human clinical trials. Search strategy and selection criteria This review was built on a systematic MEDLINE search for publications on the subject with the key words: DPP-4 inhibitor; Vildagliptin (LAF-237); sitagliptin (MK-0431); saxagliptin (BMS-477118); and type 2 diabetes; up to August 2006. Meeting abstracts were also searched, as much of the data currently only exists in abstract form. Take home message for clinician The DPP-4 inhibitors appear to have great potential for the treatment of type 2 diabetes, but time will tell if this will be realized. While they do not lower glucose to a greater extent than existing therapies, they offer many potential advantages, including the ability to achieve sustainable reductions in HbA1c with a well-tolerated agent that has a low risk of hypoglycaemia and no weight gain, and which can be administered as a once-daily oral dose.
Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats.[Pubmed:22025647]
J Pharmacol Exp Ther. 2012 Feb;340(2):248-55.
Dipeptidyl peptidase (DPP) IV inhibitors are probably beneficial for preventing diabetic complication and modulating glucagon-like peptide-1 receptor (GLP-1R) expression. The aim of this study was to determine whether the DPP IV inhibitor LAF237 (vildagliptin) has renoprotective qualities in streptozotocin-induced diabetic rats. Diabetic and nondiabetic rats were treated with an oral dose of 4 or 8 mg/kg/day LAF237 or placebo for 24 weeks, and renal injury was observed by light and electron microscopy. We also assessed DPP IV activity, active GLP-1 level, cAMP and 8-hydroxy-deoxyguanosine excretion, and GLP-1R, cleaved caspase 3, and transforming growth factor-beta1 (TGF-beta1) expression. LAF237 significantly decreased proteinuria, albuminuria, and urinary albumin/creatinine ratio, improved creatinine clearance, and dose-dependently inhibited interstitial expansion, glomerulosclerosis, and the thickening of the glomerular basement membrane in diabetic rats. It is noteworthy that LAF237 markedly down-regulated DPP IV activity and increased active GLP-1 levels, which probably prevented oxidative DNA damage and renal cell apoptosis by activating the GLP-1R and modulating cAMP. Renoprotection was also associated with a reduction in TGF-beta1 overexpression. Our study suggests that DPP IV inhibitors may ameliorate diabetic nephropathy as well as reduce the overproduction of TGF-beta1. The observed renoprotection is probably attributable to inhibition of DPP IV activity, mimicking of incretin action, and activation of the GLP-1R.
The dipeptidyl peptidase IV inhibitors vildagliptin and K-579 inhibit a phospholipase C: a case of promiscuous scaffolds in proteins.[Pubmed:25671081]
F1000Res. 2013 Dec 27;2:286.
The long term side effects of any newly introduced drug is a subject of intense research, and often raging controversies. One such example is the dipeptidyl peptidase-IV (DPP4) inhibitor used for treating type 2 diabetes, which is inconclusively implicated in increased susceptibility to acute pancreatitis. Previously, based on a computational analysis of the spatial and electrostatic properties of active site residues, we have demonstrated that phosphoinositide-specific phospholipase C (PI-PLC) from Bacillus cereus is a prolyl peptidase using in vivo experiments. In the current work, we first report the inhibition of the native activity of PI-PLC by two DPP4 inhibitors - Vildagliptin (LAF-237) and K-579. While vildagliptin inhibited PI-PLC at micromolar concentrations, K-579 was a potent inhibitor even at nanomolar concentrations. Subsequently, we queried a comprehensive, non-redundant set of 5000 human proteins (50% similarity cutoff) with known structures using serine protease (SPASE) motifs derived from trypsin and DPP4. A pancreatic lipase and a gastric lipase are among the proteins that are identified as proteins having promiscuous SPASE scaffolds that could interact with DPP4 inhibitors. The presence of such scaffolds in human lipases is expected since they share the same catalytic mechanism with PI-PLC. However our methodology also detects other proteins, often with a completely different enzymatic mechanism, that have significantly congruent domains with the SPASE motifs. The reported elevated levels of serum lipase, although contested, could be rationalized by inhibition of lipases reported here. In an effort to further our understanding of the spatial and electrostatic basis of DPP4 inhibitors, we have also done a comprehensive analysis of all 76 known DPP4 structures liganded to inhibitors till date. Also, the methodology presented here can be easily adopted for other drugs, and provide the first line of filtering in the identification of pathways that might be inadvertently affected due to promiscuous scaffolds in proteins.
Combination of dipeptidylpeptidase IV inhibitor and low dose thiazolidinedione: preclinical efficacy and safety in db/db mice.[Pubmed:17532347]
Life Sci. 2007 Jun 13;81(1):72-9.
Thiazolidinediones (TZDs) are currently the most efficacious class of oral antidiabetics. However, they carry the burden of weight gain and haemodilution, which may lead to cardiovascular complications. The present study was designed to ascertain whether a combination of dipeptidyl peptidase IV (DPP IV) inhibitor with low dose of a thiazolidinedione absolves TZD associated weight gain and oedema without compromising its efficacy. In this study, we examined the efficacy and safety of lower dose (1 mg/kg/day) of rosiglitazone, a thiazolidinedione, in combination with 5 mg/kg/day dose of LAF-237 (vildagliptin), a known DPP IV inhibitor, in aged db/db mice after 14 days of treatment and compared the combination with therapeutic dose (10 mg/kg) of rosiglitazone. The combination therapy showed similar efficacy as that of 10 mg/kg/day rosiglitazone in lowering random blood glucose (53.8%, p<0.001 and 54.3%, p<0.001 respectively), AUC ((0-120) min) during oral glucose tolerance test (OGTT) (38.6 %, p<0.01; 38.3%, p<0.01 respectively) and triglyceride levels (63.9% and 61% respectively; p<0.01). Plasma active glucagon like peptide-1 (GLP-1) and insulin levels were found to be elevated significantly (p<0.01 and p<0.05 respectively) in both LAF-237 and combination treated groups following oral glucose load. LAF-237 alone had no effect on random glucose and glucose excursion during OGTT in severely diabetic db/db mice. Interestingly, the combination treatment showed no significant increase in body weight as compared to the robust weight gain by therapeutic dose of rosiglitazone. Rosiglitazone at 10 mg/kg/day showed significant reduction (p<0.05) in haematocrit, RBC count, haemoglobin pointing towards haemodilution associated with increased mRNA expression of Na(+), K(+)-ATPase-alpha and epithelial sodium channel gamma (ENaCgamma) in kidney. The combination therapy escaped these adverse effects. The results suggest that combination of DPP IV inhibitor with low dose of thiazolidinedione can interact synergistically to represent a therapeutic advantage for the clinical treatment of type 2 diabetes without the adverse effects of haemodilution and weight gain associated with thiazolidinediones.


