T 98475GnRH receptor antagonist CAS# 199119-18-1 |
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
Quality Control & MSDS
Number of papers citing our products

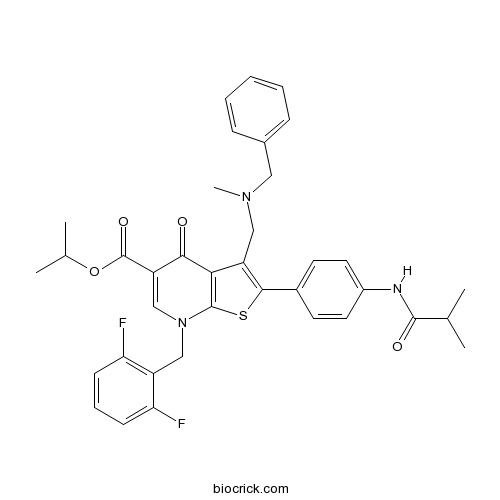
Chemical structure

3D structure
| Cas No. | 199119-18-1 | SDF | Download SDF |
| PubChem ID | 9874838 | Appearance | Powder |
| Formula | C37H37F2N3O4S | M.Wt | 657.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
| Chemical Name | propan-2-yl 3-[[benzyl(methyl)amino]methyl]-7-[(2,6-difluorophenyl)methyl]-2-[4-(2-methylpropanoylamino)phenyl]-4-oxothieno[2,3-b]pyridine-5-carboxylate | ||
| SMILES | CC(C)C(=O)NC1=CC=C(C=C1)C2=C(C3=C(S2)N(C=C(C3=O)C(=O)OC(C)C)CC4=C(C=CC=C4F)F)CN(C)CC5=CC=CC=C5 | ||
| Standard InChIKey | RANJJVIMTOIWIN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, orally active and non-peptide gonadotropin-releasing hormone (GnRH, LHRH) receptor antagonist (IC50 values are 0.2, 4.0 and 60 nM for human, monkey and rat GnRH receptors respectively). Inhibits LH release in vitro (IC50 = 100 nM) and reduces plasma LH concentration in castrated male cynomolgus monkeys. |

T 98475 Dilution Calculator

T 98475 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5203 mL | 7.6014 mL | 15.2029 mL | 30.4058 mL | 38.0072 mL |
| 5 mM | 0.3041 mL | 1.5203 mL | 3.0406 mL | 6.0812 mL | 7.6014 mL |
| 10 mM | 0.152 mL | 0.7601 mL | 1.5203 mL | 3.0406 mL | 3.8007 mL |
| 50 mM | 0.0304 mL | 0.152 mL | 0.3041 mL | 0.6081 mL | 0.7601 mL |
| 100 mM | 0.0152 mL | 0.076 mL | 0.152 mL | 0.3041 mL | 0.3801 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- H-Phe(4-Me)-OH
Catalog No.:BCC3270
CAS No.:1991-87-3
- Maackiain
Catalog No.:BCN1236
CAS No.:19908-48-6
- Bakkenolide A
Catalog No.:BCN5402
CAS No.:19906-72-0
- Dihydromethysticin
Catalog No.:BCN2476
CAS No.:19902-91-1
- Syringaresinol diacetate
Catalog No.:BCN4874
CAS No.:1990-77-8
- Kaempferol 3-O-beta-sophoroside
Catalog No.:BCN3336
CAS No.:19895-95-5
- 29-Nor-20-oxolupeol
Catalog No.:BCN6678
CAS No.:19891-85-1
- Jaborosalactone D
Catalog No.:BCN7946
CAS No.:19891-82-8
- Nagilactone B
Catalog No.:BCN4049
CAS No.:19891-51-1
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- Continentalic acid
Catalog No.:BCN6526
CAS No.:19889-23-7
- Furanodiene
Catalog No.:BCN6454
CAS No.:19912-61-9
- Chamigrenal
Catalog No.:BCN7847
CAS No.:19912-84-6
- Futoenone
Catalog No.:BCN6408
CAS No.:19913-01-0
- Enniatin B1
Catalog No.:BCN4853
CAS No.:19914-20-6
- Boc-D-N-Me-Ala-OH
Catalog No.:BCC3211
CAS No.:19914-38-6
- O6-Benzylguanine
Catalog No.:BCC6485
CAS No.:19916-73-5
- Dehydroglyasperin C
Catalog No.:BCN6790
CAS No.:199331-35-6
- Glyurallin A
Catalog No.:BCN7538
CAS No.:199331-36-7
- Crocatone
Catalog No.:BCN3532
CAS No.:19937-86-1
- Kolavenol
Catalog No.:BCN4680
CAS No.:19941-83-4
- Gymnestrogenin
Catalog No.:BCN7846
CAS No.:19942-02-0
- 3-Epicabraleadiol
Catalog No.:BCN4875
CAS No.:19942-04-2
Local Inflammatory Cues Regulate Differentiation and Persistence of CD8(+) Tissue-Resident Memory T Cells.[Pubmed:28380351]
Cell Rep. 2017 Apr 4;19(1):114-124.
Many pathogens initiate infection at mucosal surfaces, and tissue-resident memory T (Trm) cells play an important role in protective immunity, yet the tissue-specific signals that regulate Trm differentiation are poorly defined. During Yersinia infection, CD8(+) T cell recruitment to areas of inflammation within the intestine is required for differentiation of the CD103(-)CD69(+) Trm subset. Intestinal proinflammatory microenvironments have elevated interferon (IFN)-beta and interleukin-12 (IL-12), which regulated Trm markers, including CD103. Type I interferon-receptor- or IL-12-receptor-deficient T cells functioned similarly to wild-type (WT) cells during infection; however, the inability of T cells to respond to inflammation resulted in defective differentiation of CD103(-)CD69(+) Trm cells and reduced Trm persistence. Intestinal macrophages were the main producers of IFN-beta and IL-12 during infection, and deletion of CCR2(+) IL-12-producing cells reduced the size of the CD103(-) Trm population. These data indicate that intestinal inflammation drives phenotypic diversity and abundance of Trm cells for optimal tissue-specific immunity.
Clinical and prognostic significance of aberrant T-cell marker expression in 225 cases of de novo diffuse large B-cell lymphoma and 276 cases of other B-cell lymphomas.[Pubmed:28380441]
Oncotarget. 2017 May 16;8(20):33487-33500.
Expression of T-cell markers, generally investigated for immunophenotyping of T-cell lymphomas, is also observed in several types of B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL). We previously reported that CD5 expression in DLBCL is an inferior prognostic factor in the era of rituximab. However, data regarding the frequencies, histological relevance, and prognostic importance of T-cell markers other than CD5 are currently unavailable. In the present study, we comprehensively evaluated the expression of T-cell markers (CD2, CD3, CD4, CD5, CD7, and CD8) in 501 B-cell lymphomas, including 225 DLBCLs, by flow cytometry and subsequent immunohistochemistry. T-cell markers other than CD5, such as CD2, CD4, CD7, and CD8, were expressed in 27 (5%) patients, and notably, all of these cases were classified as large B-cell lymphoma subtypes: 25 DLBCLs and 2 intravascular large B-cell lymphomas. CD5 and other T-cell markers were expressed in 15% (31/225) and 10% (25/225) of DLBCL cases, respectively. Five of them co-expressed CD5 and other T-cell markers. Retrospectively analyzing the prognostic relevance of T-cell markers in 169 patients with primary DLBCL treated with rituximab-based chemotherapy, we showed that only CD5 was a strong predictor of poor survival. This study provides information about the occurrence of T-cell markers other than CD5 in B-cell lymphomas, their frequent histological subtypes, and their prognostic significance in DLBCL. CD5 was reconfirmed as a negative prognostic marker in DLBCL patients receiving rituximab-inclusive chemotherapy, whereas T-cell markers other than CD5 were found to have no impact on clinicopathological and survival analyses.
Hemodialysis Patients Display a Declined Proportion of Th2 and Regulatory T Cells in Parallel with a High Interferon-gamma Profile.[Pubmed:28380480]
Nephron. 2017;136(3):254-260.
BACKGROUND: A high prevalence of cardiovascular diseases (CVDs) and infections in patients with chronic kidney disease (CKD) arises partly due to a high inflammatory state and aberrations in immune cells function. Following in vitro stimulation of leukocytes with different T-cell mitogens, we observed a lower level of interleukin (IL)-2 and IL-10 production in CKD patients. To gain more knowledge as to whether this is the result of an alteration in T-cell function, we investigated the T-cell subsets profile and cytokine production in hemodialysis patients. METHODS: CD4+ cells were isolated from whole blood of 10 hemodialysis patients and 10 age- and gender-matched healthy controls. Following in vitro stimulation with an antigen-independent T-cell mitogen, Th1, Th2, and regulatory T (Treg) cell subsets were analyzed by flow cytometry through the expression of specific transcription factors. The levels of cytokines, interferon (IFN)-gamma, IL-4, and IL-10 were analyzed by enzyme-linked immunosorbent assay in the supernatants. RESULTS: The proportion of CD4+CD25+FOXP3+ (Treg) and CD4+GATA3+ (Th2) cells was significantly lower in patients compared to healthy controls, while the proportion of CD4+T-bet+ (Th1) cells was similar. Moreover, levels of IL-4 were significantly lower in supernatants from patients, while IFN-gamma levels were higher. IL-10 levels did not differ compared to those of the healthy controls. CONCLUSIONS: Our findings indicate a diminished anti-inflammatory Treg, and Th2 cell profile in hemodialysis patients, accompanied by a high pro-inflammatory IFN-gamma profile. Since this profile is characterized in CVDs, we propose that an imbalance between the inflammatory and anti-inflammatory responses may contribute to the pathogenesis of CVD in advanced CKD.
Wnt5a and CCL25 promote adult T-cell acute lymphoblastic leukemia cell migration, invasion and metastasis.[Pubmed:28380463]
Oncotarget. 2017 Jun 13;8(24):39033-39047.
Adult T-cell acute lymphoblastic leukemia (T-ALL) is a refractory leukemia. We previously showed that CCL25/CCR9 promotes T-ALL metastasis. In the present study, we assessed the effects of CCL25 on Wnt expression and the effects of Wnt5a and CCL25 on PI3K/Akt and RhoA activation. Transwell assays and mouse xenograft experiments were utilized to assess the effects of Wnt5a and CCL25 on MOLT4 cell invasion, migration and metastasis. The effects of Wnt5a on MOLT4 cell actin polarization and pseudopodium formation were examined using laser scanning confocal microscopy and scanning electron microscopy. CCL25 induced Wnt5a expression in MOLT4 cells by promoting protein kinase C (PKC) expression and activation. Wnt5a promoted MOLT4 cell migration, invasion, actin polarization, and lamellipodium and filopodia formation via PI3K/Akt-RhoA pathway activation. These effects were rescued by PI3K/Akt or RhoA knockdown or inhibition. Additionally, Wnt5a in cooperation with CCL25 promoted MOLT4 cell mouse liver metastasis and stimulated RhoA activation. These results show that CCL25/CCR9 upregulates Wnt5a by promoting PKC expression and activation in MOLT4 cells. This in turn promotes cell migration and invasion via PI3K/Akt-RhoA signaling, enhancing cell polarization and pseudopodium formation. These findings indicate that the PI3K/Akt-RhoA pathway is likely responsible for Wnt5a-induced adult T-ALL cell migration and invasion.
Design, synthesis, and structure-activity relationships of thieno[2,3-b]pyridin-4-one derivatives as a novel class of potent, orally active, non-peptide luteinizing hormone-releasing hormone receptor antagonists.[Pubmed:16789738]
J Med Chem. 2006 Jun 29;49(13):3809-25.
Design, synthesis, and structure-activity relationships of thieno[2,3-b]pyridin-4-one-based non-peptide luteinizing hormone-releasing hormone (LHRH) receptor antagonists are described. Starting with the thienopyridin-4-one derivative 26d (T-98475) an optimization study was performed, which resulted in the identification of a highly potent and orally bioavailable LHRH receptor antagonist, 3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobenzyl)-4,7-dihydro-2-[4-(1-hydro xy-1-cyclopropanecarboxamido)phenyl]-5-isobutyryl-4-oxothieno[2,3-b]pyridine (33c). Compound 33c displayed subnanomolar in vitro activities for the human receptor and its oral administration caused effective suppression of the plasma LH levels in castrated male cynomolgus monkeys. Furthermore, SAR studies revealed that a hydroxyalkylamido moiety on the 2-phenyl ring is virtually equivalent to an alkylureido moiety, at least in this series of compounds.
Discovery of a thieno[2,3-d]pyrimidine-2,4-dione bearing a p-methoxyureidophenyl moiety at the 6-position: a highly potent and orally bioavailable non-peptide antagonist for the human luteinizing hormone-releasing hormone receptor.[Pubmed:12502365]
J Med Chem. 2003 Jan 2;46(1):113-24.
We have previously disclosed the first potent and orally effective non-peptide antagonist for the human luteinizing hormone-releasing hormone (LHRH) receptor, a thieno[2,3-b]pyridin-4-one derivative, T-98475 (1). Extensive research on developing non-peptide LHRH antagonists has been carried out by employing a strategy of replacing the thienopyridin-4-one nucleus with other heterocyclic surrogates. We describe herein the design and synthesis of a series of thieno[2,3-d]pyrimidine-2,4-dione derivatives containing a biaryl moiety, which led to the discovery of a highly potent and orally active non-peptide LHRH antagonist, 5-(N-benzyl-N-methylaminomethyl)-1-(2,6-difluorobenzyl)-6-[4-(3-methoxyureido)phe nyl]-3-phenylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione (9k: TAK-013). Compound 9k showed high binding affinity and potent in vitro antagonistic activity for the human receptor with half-maximal inhibition concentration (IC(50)) values of 0.1 and 0.06 nM, respectively. Oral administration of 9k caused almost complete suppression of the plasma LH levels in castrated male cynomolgus monkeys at a 30 mg/kg dose with sufficient duration of action (more than 24 h). The results demonstrated that the thienopyrimidine-2,4-dione core is an excellent surrogate for the thienopyridin-4-one and that thienopyrimidine-2,4-diones and thienopyridin-4-ones constitute a new class of potent and orally bioavailable LHRH receptor antagonists. Furthermore, molecular modeling studies indicate that the unique methoxyurea side chain of 9k preferentially forms an intramolecular hydrogen bond between the aniline NH and the methoxy oxygen atom. The hydrogen bond will shield the hydrogen bonding moieties from the solvent and reduce the desolvation energy cost. It is therefore speculated that the intramolecular hydrogen bond resulting from judicious incorporation of an oxygen atom into the terminal alkyl group of the urea may increase the apparent lipophilicity to allow increased membrane permeability and consequently to improve the oral absorption of 9k in monkeys. On the basis of its profile, compound 9k has been selected as a candidate for clinical trials and it is expected that it will provide a new class of potential therapeutic agents for the clinical treatment of a variety of sex-hormone-dependent diseases.


