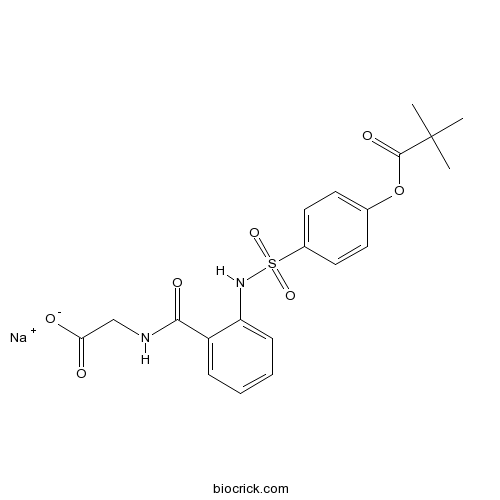
Sivelestat sodium saltLeukocyte elastase inhibitor CAS# 150374-95-1 |
- Atrasentan
Catalog No.:BCC1379
CAS No.:173937-91-2
- Zibotentan (ZD4054)
Catalog No.:BCC2524
CAS No.:186497-07-4
- Atrasentan hydrochloride
Catalog No.:BCC1380
CAS No.:195733-43-8
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Macitentan
Catalog No.:BCC1142
CAS No.:441798-33-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 150374-95-1 | SDF | Download SDF |
| PubChem ID | 23664980 | Appearance | Powder |
| Formula | C20H21N2NaO7S | M.Wt | 456.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ONO-5046 | ||
| Solubility | DMSO : 100 mg/mL (219.09 mM; Need ultrasonic) H2O : 1 mg/mL (2.19 mM; ultrasonic and warming and heat to 80°C) | ||
| Chemical Name | sodium;2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetate | ||
| SMILES | CC(C)(C)C(=O)OC1=CC=C(C=C1)S(=O)(=O)NC2=CC=CC=C2C(=O)NCC(=O)[O-].[Na+] | ||
| Standard InChIKey | ZAIFANJZUGNYCK-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C20H22N2O7S.Na/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24;/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective leukocyte elastase inhibitor (IC50 = 44 nM) that displays no activity at a range of other proteases. Inhibits NF-κB activation and LTB4-induced neutrophil transmigration in vitro. Significantly attenuates ischemia-induced spinal cord injury, decreases serum cytokine levels and reduces acute inflammatory lung injury in vivo. |

Sivelestat sodium salt Dilution Calculator

Sivelestat sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1909 mL | 10.9543 mL | 21.9087 mL | 43.8174 mL | 54.7717 mL |
| 5 mM | 0.4382 mL | 2.1909 mL | 4.3817 mL | 8.7635 mL | 10.9543 mL |
| 10 mM | 0.2191 mL | 1.0954 mL | 2.1909 mL | 4.3817 mL | 5.4772 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4382 mL | 0.8763 mL | 1.0954 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.2191 mL | 0.4382 mL | 0.5477 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective leukocyte elastase inhibitor (IC50 = 44 nM) that displays no activity at a range of other proteases. Inhibits NF-κB activation and LTB4-induced neutrophil transmigration in vitro. Significantly attenuates ischemia-induced spinal cord injury, decreases serum cytokine levels and reduces acute inflammatory lung injury in vivo.
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Prasugrel
Catalog No.:BCC1089
CAS No.:150322-43-3
- Z-Aib-OH
Catalog No.:BCC3150
CAS No.:15030-72-5
- H-Aib-Ome.HCl
Catalog No.:BCC2671
CAS No.:15028-41-8
- Ledipasvir D-tartrate
Catalog No.:BCC4047
CAS No.:1502654-87-6
- Fmoc-p-amino-benzoic acid,Fmoc-4-Abz-OH
Catalog No.:BCC2622
CAS No.:15026-42-1
- Fmoc-2-Abz-OH
Catalog No.:BCC3204
CAS No.:150256-42-1
- 12-Deoxo-12alpha-acetoxyelliptone
Catalog No.:BCN4803
CAS No.:150226-21-4
- Trigothysoid N
Catalog No.:BCN6881
CAS No.:1501943-08-3
- BIM 23056
Catalog No.:BCC5824
CAS No.:150155-61-6
- SR 49059
Catalog No.:BCC7324
CAS No.:150375-75-0
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- L-NIL hydrochloride
Catalog No.:BCC5706
CAS No.:150403-89-7
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- Fmoc-Lys(Dde)-OH
Catalog No.:BCC3518
CAS No.:150629-67-7
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
A pilot randomized study of the neutrophil elastase inhibitor, Sivelestat, in patients undergoing cardiac surgery.[Pubmed:19447800]
Interact Cardiovasc Thorac Surg. 2009 Aug;9(2):236-40.
The primary objective of this study was to determine the feasibility and safety of treatment with Sivelestat in humans to attenuate post-cardiopulmonary bypass lung injury. Twenty patients scheduled to undergo on-pump coronary artery bypass surgery were randomized to receive either 0.3 mg/kg/h intravenous Sivelestat sodium (Sivelestat group; n=10) or isotonic sodium chloride solution (placebo group, n=10), peri-operatively. Postoperative adverse events were recorded until hospital discharge. The alveolar-arterial oxygen gradient (A-aDO(2)), intrapulmonary shunt (Qs/Qt) and dynamic lung compliance were determined four times peri-operatively as secondary exploratory outcomes. All patients completed study protocol without discontinuation of intervention. The number of total adverse clinical outcomes, including atrial fibrillation and superficial wound infection, was nine in seven patients in the placebo group and four in four patients in the Sivelestat group (P=0.37). The mean duration of the postoperative hospital stay was shorter in the Sivelestat group (19.0+/-3.4 vs. 25.6+/-9.1, P=0.04). The exploratory analysis of relative changes in lung functions showed trends toward attenuation of lung injury in the Sivelestat group in all three pulmonary parameters, though the inter-group difference could be due to chance (P>0.05). It is feasible to administer Sivelestat as a preventive measure against lung dysfunction after cardiopulmonary bypass.
Gateways to clinical trials.[Pubmed:21225012]
Methods Find Exp Clin Pharmacol. 2010 Dec;32(10):749-73.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Thomson Reuters Integrity(SM), the drug discovery and development portal, http://www.thomsonreutersintegrity.com. This issue focuses on the following selection of drugs: 17-Hydroxyprogesterone caproate; Abacavir sulfate/lamivudine, Aclidinium bromide, Adalimumab, Adefovir, Alemtuzumab, Alkaline phosphatase, Amlodipine, Apilimod mesylate, Aripiprazole, Axitinib, Azacitidine; Belotecan hydrochloride, Berberine iodide, Bevacizumab, Bortezomib, Bosentan, Bryostatin 1; Calcipotriol/hydrocortisone, Carglumic acid, Certolizumab pegol, Cetuximab, Cinacalcet hydrochloride, Cixutumumab, Coumarin, Custirsen sodium; Darbepoetin alfa, Darifenacin hydrobromide, Darunavir, Dasatinib, Denibulin hydrochloride, Denosumab, Diacetylmorphine, Dulanermin, Duloxetine hydrochloride; Ecogramostim, Enfuvirtide, Entecavir, Enzastaurin hydrochloride, Eplerenone, Escitalopram oxalate, Esomeprazole sodium, Etravirine, Everolimus, Ezetimibe; Fenofibrate/pravastatin sodium, Ferric carboxymaltose, Flavangenol, Fondaparinux sodium; Glutamine, GSK-1024850A; Hepatitis B hyperimmunoglobulin, Hib-MenC, HIV-LIPO-5; Immunoglobulin intravenous (human), Indacaterol maleate, Indibulin, Indium 111 ((1)(1)(1)In) ibritumomab tiuxetan, Influenza A (H1N1) 2009 Monovalent vaccine, Inhalable human insulin, Insulin glulisine; Lapatinib ditosylate, Leucovorin/UFT; Maraviroc, Mecasermin, MMR-V, Morphine hydrochloride, Morphine sulfate/naltrexone hydrochloride, Mycophenolic acid sodium salt; Naproxen/esomeprazole magnesium, Natalizumab; Oncolytic HSV; Paliperidone, PAN-811, Paroxetine, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b/ribavirin, Pegvisomant, Pemetrexed disodium, Pimecrolimus, Posaconazole, Pregabalin; Raltegravir potassium, Ranelic acid distrontium salt, Rasburicase, Rilpivirine hydrochloride; Sertindole, Sivelestat sodium hydrate, Sorafenib, Sumatriptan succinate/naproxen sodium, Sunitinib malate; Tafluprost, Telithromycin, Temsirolimus, Tenofovir disoproxil fumavate, Tenofovir disoproxil fumarate/emtricitabine, Teriparatide, Ticagrelor, Tigecycline, Tipranavir, Tirapazamine, Trimetrexate; Ulipristal acetate; Valganciclovir hydrochloride, Vicriviroc, Vorinostat; Yttrium 90 (90Y) ibritumomab tiuxetan.
Gateways to clinical trials.[Pubmed:19455266]
Methods Find Exp Clin Pharmacol. 2009 Mar;31(2):107-46.
ABT-869, Acadesine, Acetylsalicylic acid/omeprazole, Adefovir, Adefovir dipivoxil, AEG-35156, Agatolimod sodium, Albiglutide, Alemtuzumab, Alipogene tiparvovec, Alogliptin benzoate, AMG-386, Amrubicin hydrochloride, Apremilast, Aripiprazole, Asoprisnil, Atorvastatin/fenofibrate, AVN-944, Axitinib; Belinostat, Bevacizumab, BHT-3021, BI-2536, Biapenem, Bilastine, Biphasic insulin aspart, Blinatumomab, Bortezomib, Bosentan; Catumaxomab, CD-NP, Cediranib, Certolizumab pegol, Cetuximab, Choline fenofibrate, Ciclesonide, CK-1827452,Clevudine, Clofarabine, CSL-360, CYT-997; Dapagliflozin, Darinaparsin, Denosumab, Densiron 68, Desloratadine, Dulanermin; Edoxaban tosilate, Emtricitabine, Entecavir, Erlotinib hydrochloride, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Fidaxomicintiacumiv, Fulvestrant; G-207, GCR-8015, Gefitinib, Ghrelin (human), Glufosfamide; HPV16L1E7CVLP; Ibutamoren mesilate, Imatinib mesylate, Insulin detemir, Insulin glargine, Iodine (I131) tositumomab, Istaroxime, ITMN-191, Ixabepilone; JZP-4, Lenalidomide; Levetiracetam, Linaclotide acetate, Liposomal cytarabine/daunorubicin, Liposomal doxorubicin, Liraglutide, LY-518674; Milatuzumab, MMR-V, Motesanib diphosphate, Mycophenolic acid sodium salt; Niacin/simvastatin; Obatoclax mesylate, Odanacatib; Paclitaxel nanoparticles, Paclitaxel-eluting stent, Pazufloxacin, PBT-2, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ribavirin, Pemetrexed disodium, Perampanel, PfCP2.9, Pitavastatin calcium, Poly I:CLC, Pomalidomide, Pralatrexate, Pramlintide acetate, Prucalopride; rhGAD65, Roflumilast; RTS,S/AS02D; SCH-530348, Semagacestat, Sirolimus-eluting coronary stent, Sirolimus-Eluting Stent, SIR-Spheres, Sivelestat sodium hydrate, Sorafenib, Sunitinib malate; Tadalafil, Tafluprost, Tanespimycin, Teduglutide, Telaprevir, Telbivudine, Tenofovir disoproxil fumarate, Tiotropium bromide, TMC-435350, Tositumomab/iodine (I131) tositumomab, Travoprost/timolol, Triciribine phosphate; Vandetanib, VIA-2291, Vinflunine, Vorinostat; XL-019; Yttrium 90 (90Y) ibritumomab tiuxetan.
Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NF-kB.[Pubmed:19169649]
Inflamm Res. 2009 Apr;58(4):198-203.
OBJECTIVE: Sivelestat sodium hydrate (sivelestat) is a specific synthetic inhibitor of neutrophil elastase (NE). Various studies suggest that sivelestat treatment reduces inflammation. In this study, we tested the hypothesis that sivelestat acts as an inhibitor of inflammatory mediators and prevents nuclear factor-kB (NF-kB) activation. METHODS: In the presence and absence of sivelestat, the mouse macrophage cell line RAW 264.7 was stimulated with lipopolysaccharide (LPS) and the levels of inflammatory mediators (TNF-alpha, IL-6 and high mobility group box 1 (HMGB1)) and nitrite in the cell supernatant were measured, along with inducible nitric oxide synthase (iNOS) expression. RESULTS: While LPS administration increased the secretion of inflammatory mediators and nitric oxide (NO), sivelestat decreased the secretion of these mediators. Cell signaling studies demonstrated that sivelestat decreased NF-kB activation by inhibiting IkB phosphorylation. CONCLUSION: Sivelestat may inhibit the various inflammatory mediators through NF-kB inhibition.
Protective effect of sivelestat sodium hydrate (ONO-5046) on ischemic spinal cord injury.[Pubmed:19289399]
Interact Cardiovasc Thorac Surg. 2009 Jun;8(6):606-9.
Prevention of paraplegia remains an important issue in repair of descending thoracic and thoracoabdominal aneurysms. Therefore, we investigated the protective effect of sivelestat sodium hydrate (ONO-5046) on ischemia-induced spinal cord damage in a rabbit model. Twenty New Zealand white rabbits were divided into two equal groups; ONO-5046 (1.6 mg/kg)+isotonic NaCl (30 ml) was administered selectively to the spinal cord via the lumbar arteries for the first 3 min during 30 min of infra-renal aorta clamping in the experimental group (group E), whereas NaCl was given alone in the control group (group C). Motor function of the lower limbs was assessed two days later by Tarlov criteria. The number of intact motor neurons in the anterior segment of the cord (L5 level) was counted after hematoxylin-eosin staining and the number of apoptotic motor neurons after TUNEL staining. Motor function of the lower limbs in group E was significantly better (P=0.003) than that in group C. The number of intact motor neurons was greater and of apoptotic motor neurons was less in group E than C. Selective infusion of sivelestat sodium hydrate directly into the spinal cord via the lumbar arteries significantly attenuated functional and morphological ischemia-induced spinal cord injury.
Role of neutrophil elastase in LTB4-induced neutrophil transmigration in vivo assessed with a specific inhibitor and neutrophil elastase deficient mice.[Pubmed:17471175]
Br J Pharmacol. 2007 Jul;151(5):628-37.
BACKGROUND AND PURPOSE: The serine protease neutrophil elastase (NE) appears to regulate inflammatory responses at multiple levels but its role in leukocyte transmigration in vivo remains unclear. The present study aimed to address this issue by using both an NE inhibitor (ONO-5046) and NE deficient (NE(-/-)) mice. EXPERIMENTAL APPROACH: A number of inflammatory mediators (LTB(4), KC and PAF) were investigated in vitro for their ability to stimulate the release and the surface expression of NE by neutrophils. In addition, the role of NE in leukocyte migration elicited by topical LTB(4) was investigated in vivo in mouse cremasteric venules as observed by intravital microscopy. KEY RESULTS: Amongst the mediators tested in vitro, LTB(4) was found to be a highly potent and efficacious inducer of NE cell surface expression on murine neutrophils. Furthermore, in wild-type mice (WT), LTB(4)-induced leukocyte transmigration was reduced by intravenous ONO-5046 (66% inhibition), an effect that appeared to occur at the level of the perivascular basement membrane. Interestingly, LTB(4)-induced responses were normal in NE(-/-) mice and, while ONO-5046 had no inhibitory effect in these animals, the broad-spectrum serine protease inhibitor aprotinin suppressed leukocyte transmigration in both WT and NE(-/-) mice. CONCLUSIONS AND IMPLICATIONS: The findings demonstrate the potent ability of LTB(4) to induce cell-surface expression of NE and provide evidence for the involvement of NE in LTB(4)-induced neutrophil transmigration in vivo. The results also suggest the existence of compensatory mechanisms in NE(-/-) mice, highlighting the added value of investigating pharmacological blockers in parallel with genetic deletion.
ONO-5046, a novel inhibitor of human neutrophil elastase.[Pubmed:2049103]
Biochem Biophys Res Commun. 1991 Jun 14;177(2):814-20.
ONO-5046, N-[2-[4-(2,2-Dimethylpropionyloxy)phenylsulfonylamino] aminoacetic acid, competitively inhibited human neutrophil elastase (IC50 = 0.044 microM, Ki = 0.2 microM). It also inhibited leukocyte elastase obtained from rabbit, rat, hamster and mouse. However, ONO-5046 did not inhibit trypsin, thrombin, plasmin, plasma kallikrein, pancreas kallikrein, chymotrypsin and cathepsin G even at 100 microM. In in vivo studies, ONO-5046 suppressed lung hemorrhage in hamster (ID50 = 82 micrograms/kg) by intratracheal administration and increase of skin capillary permeability in guinea pig (ID50 = 9.6 mg/kg) by intravenous administration, both of which were induced by human neutrophil elastase.


