SimeprevirInhibitor of HCV NS3/4A protease CAS# 923604-59-5 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure
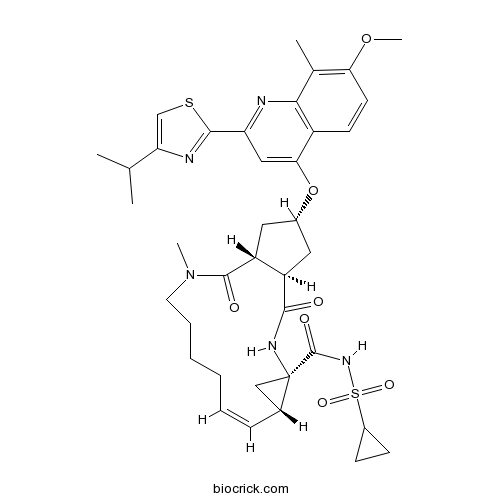
3D structure
| Cas No. | 923604-59-5 | SDF | Download SDF |
| PubChem ID | 24873435 | Appearance | Powder |
| Formula | C38H47N5O7S2 | M.Wt | 749.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TMC435 | ||
| Solubility | DMSO : 14.29 mg/mL (19.05 mM; Need ultrasonic) | ||
| Chemical Name | (1R,4R,6S,7Z,15R,17R)-N-cyclopropylsulfonyl-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carboxamide | ||
| SMILES | CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)NS(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC | ||
| Standard InChIKey | JTZZSQYMACOLNN-VDWJNHBNSA-N | ||
| Standard InChI | InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-35(40-30)29-18-32(26-13-14-31(49-5)22(3)33(26)39-29)50-24-16-27-28(17-24)36(45)43(4)15-9-7-6-8-10-23-19-38(23,41-34(27)44)37(46)42-52(47,48)25-11-12-25/h8,10,13-14,18,20-21,23-25,27-28H,6-7,9,11-12,15-17,19H2,1-5H3,(H,41,44)(H,42,46)/b10-8-/t23-,24-,27-,28-,38-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Simeprevir is a potent HCV NS3/4A protease inhibitor, and inhibits HCV replication with EC50 of 8 nM.In Vitro:In Huh7-Luc cells, antiviral activity of simeprevir (TMC435350) is dose dependent, and the EC50 and EC90 values determined for TMC435350 are 8 nM and 24 nM, respectively. Inhibition of TMC435350 on NS3/4A protease is time dependent, and the overall Kis are estimated to be 0.5 nM for genotype 1a and 0.4 nM for genotype 1b, respectively[1]. TMC435350 is a potent inhibitor of HCV NS3/4A protease (Ki=0.36 nM) and viral replication (replicon EC50=7.8 nM)[2].In Vivo:In rats, TMC435350 (40 mg/kg, p.o.) is extensively distributed to the liver and intestinal tract (tissue/plasma area under the concentration-time curve ratios of >35), and the absolute bioavailability is 44%[1]. References: | |||||

Simeprevir Dilution Calculator

Simeprevir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3334 mL | 6.667 mL | 13.334 mL | 26.6681 mL | 33.3351 mL |
| 5 mM | 0.2667 mL | 1.3334 mL | 2.6668 mL | 5.3336 mL | 6.667 mL |
| 10 mM | 0.1333 mL | 0.6667 mL | 1.3334 mL | 2.6668 mL | 3.3335 mL |
| 50 mM | 0.0267 mL | 0.1333 mL | 0.2667 mL | 0.5334 mL | 0.6667 mL |
| 100 mM | 0.0133 mL | 0.0667 mL | 0.1333 mL | 0.2667 mL | 0.3334 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Simeprevir is a potent inhibitor of HCV NS3/4A protease with Ki value of 0.36 nM [1].
The hepatitis C virus (HCV) NS3/4A protease is a serine protease encoded by HCV and is responsible for cleavage at four sites of the HCV polyprotein. It is essential for viral replication [1].
In Huh-7-Rep cells, Simeprevir inhibited HCV replication with EC50 of 7.8 nM [1]. In the Huh7-Luc HCV genotype 1b replicon cell line, Simeprevir inhibited HCV replication with EC50 and EC90 values of 8 nM and 24 nM respectively in a dose dependent way [2].
In ninety-two naive, HCV genotype 1-infected patients, treatment with Simeprevir (50 or 100 mg QD) for 12 or 24 weeks and peginterferon α-2a/ribavirin (PegIFNα-2a/RBV) for 24 or 48 weeks or PegIFN α-2a/RBV for 48 weeks lonely (PR48 group), RNA reductions in the 4 week were -5.2,-5.2 and-2.9 log10IU/mL for Simeprevir 50 mg combined, 100 mg combined and PR48 groups, respectively, which suggested Simeprevir reduced HCV RNA more rapidly and substantially. Also, Simeprevir increased the virologic response rates [3].
References:
[1]. Raboisson P, de Kock H, Rosenquist A, et al. Structure-activity relationship study on a novel series of cyclopentane-containing macrocyclic inhibitors of the hepatitis C virus NS3/4A protease leading to the discovery of TMC435350. Bioorg Med Chem Lett, 2008, 18(17): 4853-4858.
[2]. Lin TI, Lenz O, Fanning G, et al. In vitro activity and preclinical profile of TMC435350, a potent hepatitis C virus protease inhibitor. Antimicrob Agents Chemother, 2009, 53(4): 1377-1385.
[3]. Hayashi N, Seto C, Kato M, et al. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol, 2014, 49(1): 138-147.
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
- GSK962040 hydrochloride
Catalog No.:BCC4152
CAS No.:923565-22-4
- GSK962040
Catalog No.:BCC4163
CAS No.:923565-21-3
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
- SAR407899
Catalog No.:BCC5593
CAS No.:923359-38-0
- Nilotinib monohydrochloride monohydrate
Catalog No.:BCC1801
CAS No.:923288-90-8
- Opicapone
Catalog No.:BCC6545
CAS No.:923287-50-7
- SAR407899 hydrochloride
Catalog No.:BCC5592
CAS No.:923262-96-8
- Refametinib R enantiomer
Catalog No.:BCC4055
CAS No.:923032-38-6
- Refametinib
Catalog No.:BCC4276
CAS No.:923032-37-5
- 3-O-Methyl-3-methoxymaxterone
Catalog No.:BCC8639
CAS No.:92282-70-7
- Sanggenon N
Catalog No.:BCN4846
CAS No.:92280-12-1
- 3-Acetoxy-4-cadinen-8-one
Catalog No.:BCN4461
CAS No.:923950-05-4
- DUBs-IN-3
Catalog No.:BCC5258
CAS No.:924296-17-3
- DUBs-IN-1
Catalog No.:BCC5256
CAS No.:924296-18-4
- DUBs-IN-2
Catalog No.:BCC5257
CAS No.:924296-19-5
- HBX 41108
Catalog No.:BCC6137
CAS No.:924296-39-9
- AdipoRon
Catalog No.:BCC4756
CAS No.:924416-43-3
- T 5601640
Catalog No.:BCC5617
CAS No.:924473-59-6
- AZD-5597
Catalog No.:BCC6453
CAS No.:924641-59-8
- 4,5-Epoxyartemisinic acid
Catalog No.:BCN4462
CAS No.:92466-31-4
- Rupesin E
Catalog No.:BCN7009
CAS No.:924901-58-6
- 21-Deoxyneridienone B
Catalog No.:BCN4463
CAS No.:924910-83-8
- Viscidulin III
Catalog No.:BCN4464
CAS No.:92519-91-0
Efficacy, safety, and pharmacokinetics of simeprevir, daclatasvir, and ribavirin in patients with recurrent hepatitis C virus genotype 1b infection after orthotopic liver transplantation: The Phase II SATURN study.[Pubmed:28295849]
Transpl Infect Dis. 2017 Jun;19(3).
BACKGROUND: Recurrent hepatitis C virus (HCV) infection following liver transplantation is associated with accelerated progression to graft failure and reduced patient survival. METHODS: The Phase II, open-label SATURN study (NCT01938625) investigated the combination of Simeprevir (SMV), daclatasvir (DCV), and ribavirin (RBV) administered for 24 weeks in 35 patients with recurrent HCV genotype (GT) 1b infection after orthotopic liver transplantation (OLT). RESULTS: High rates of both on-treatment and sustained virologic response 12 weeks after end of treatment (SVR12) were achieved in patients who were either treatment-naive or had failed post-OLT treatment with peginterferon and RBV. Overall, 91% of patients (32/35) achieved SVR12. The combination was generally well tolerated, with an adverse event profile consistent with that observed in previous clinical trials of SMV or DCV separately. Co-administration of SMV with cyclosporine resulted in significantly increased SMV plasma exposures, which was not the case with the co-administration of SMV with tacrolimus. Therefore, the concomitant use of SMV with cyclosporine is not recommended. CONCLUSION: The interferon-free combination of SMV, DCV, and RBV administered for 24 weeks was shown to be effective and well tolerated in the treatment of post-OLT HCV GT1b-infected patients.
Sofosbuvir-Daclatasvir-Simeprevir Plus Ribavirin in Direct-Acting Antiviral-Experienced Patients With Hepatitis C.[Pubmed:28369411]
Clin Infect Dis. 2017 Jun 1;64(11):1615-1618.
We assessed the broadly used, off-label combination of sofosbuvir, daclatasvir, Simeprevir, and ribavirin in direct-acting antiviral-experienced patients, as recommended in current guidelines despite scarce data. After 24 weeks' treatment, sustained virological response 12 weeks after the end of treatment was achieved in 6 patients (60%). Two cirrhotic patients relapsed and 2 discontinued treatment due to serious adverse events.
Efficacy and safety of telaprevir- and simeprevir-based triple therapies for older patients with chronic hepatitis C.[Pubmed:28261382]
World J Hepatol. 2017 Feb 18;9(5):252-262.
AIM: To evaluate and compare the efficacy and safety of telaprevir (TVR)-and Simeprevir (SMV)-based triple therapies in elderly patients, specifically patients aged 66 years or older. METHODS: The present study enrolled 112 and 76 Japanese patients with chronic hepatitis C virus genotype 1b infection who were treated with a 12-wk TVR-based or SMV-based triple therapy, respectively, followed by a dual therapy that included pegylated interferon alpha and ribavirin (RBV) for 12 wk. The patients were categorized into two groups according to age as follows: A younger group of patients aged 65 years old. Among the patients treated with TVR-based triple therapy, 34 patients were included in the older group. The median ages were 56 years (range: 28-65 years) in the younger group and 69 years (range: 66-81 years) in the older group. Among the patients treated with SMV-based triple therapy, 39 patients were included in the older group. The median ages were 59 years (range: 36-65 years) in the younger group and 71 years (range: 66-86 years) in the older group. The clinical, biochemical and virological data were analyzed before and during treatment. RESULTS: Among the patients treated with the TVR-based triple therapy, no significant difference in the sustained virological response (SVR) was found between the younger (80.8%) and older (88.2%) groups. The SVR rates for patients with the interleukin 28B (IL28B) (rs8099917) TG/GG-genotypes (73.9% and 60.0% in the younger and older groups, respectively) were significantly lower than for patients with the IL28B TT-genotype (86.3% and 92.9%, respectively). The cumulative exposure to RBV for the entire 24-wk treatment period (as a percentage of the target dose) was significantly higher in the younger group than in the older group (91.7% vs 66.7%, respectively, P < 0.01), but the cumulative exposure to TVR was not significantly different between the younger and older groups (91.6% vs 81.9%, respectively). A multivariate analysis identified the TT-genotype of IL28B (OR = 8.160; 95%CI: 1.593-41.804, P = 0.012) and the adherence of RBV (> 60%) (OR = 11.052; 95%CI: 1.160-105.273, P = 0.037) as independent factors associated with the SVR. Adverse events resulted in discontinuation of the treatment in 11.3% and 14.7% of the younger and older groups, respectively. Among the patients treated with the SMV-based triple therapy, no significant difference in the SVR rare was found between the younger (81.1%) and older (82.1%) groups. The SVR rates for patients with the IL28B TG/GG-genotypes (77.8% and 64.7% in the younger and older groups, respectively) were significantly lower than for patients with the IL28B TT-genotype (88.2% and 100%, respectively). A multivariate analysis identified the TT-genotype of IL28B as an independent factor associated with the SVR (OR = 9.677; 95%CI: 1.114-84.087, P = 0.040). Adverse events resulted in discontinuation of the treatment in 7.0% and 14.3% of patients in the younger and older groups, respectively. CONCLUSION: Both TVR- and SMV-based triple therapies can be successfully used to treat patients aged 66 years or older with genotype 1b chronic hepatitis C. Genotyping of the IL28B indicates a potential to achieve SVR in these difficult-to-treat elderly patients.
Aplastic Anemia and Severe Myelosuppression with Boceprevir or Simeprevir-Containing Hepatitis C Virus Treatment.[Pubmed:28233734]
Ann Hepatol. 2017 March-April;16(2):312-317.
The addition of the new protease inhibitors (PIs) to peg-interferon (IFN) and ribavirin (RBV), approved for chronic hepatitis C, has clearly improved sustained virological response (SVR) rates although several adverse events have been reported with this regimens, including mild hematological toxicity. Moreover, severe pancytopenia and aplastic anemia during triple therapy with telaprevir has recently been described in seven patients. We report here two cases of severe agranulocytosis/aplastic anemia using boceprevir or Simeprevir in interferon-based combination and 2 additional cases of severe myelosupression in IFN-free therapy with sofosbuvir and Simeprevir plus RBV. Our observations suggest that PIs could have a sort of class-effect in developing severe hematologic toxicity or, at least, an additive interaction with other potentially myelotoxic agents such as IFN or RBV that are used in the classical regimens against HCV. Unfortunately, the mechanisms behind this phenomenon are currently unknown. In conclusion, given the lifethreatening character of these complications, close monitoring is mandatory in patients under PIs based therapy to promptly detect serious hematological toxicities and to carefully evaluate treatment discontinuation. Prospective studies assessing the usefulness of RBV in the era of new IFN-free combinations are needed.


