Serotonin HClEndogenous substrate CAS# 153-98-0 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 153-98-0 | SDF | Download SDF |
| PubChem ID | 160436 | Appearance | Powder |
| Formula | C10H13ClN2O | M.Wt | 212.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxytryptamine, 5-HT | ||
| Solubility | DMSO : 130 mg/mL (611.25 mM; Need ultrasonic) | ||
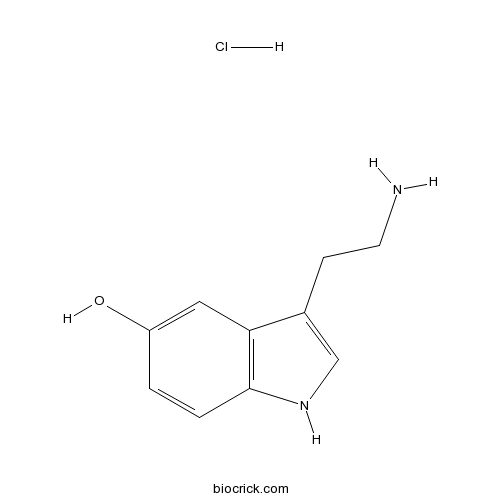
| Chemical Name | 3-(2-aminoethyl)-1H-indol-5-ol;hydrochloride | ||
| SMILES | [H+].[Cl-].NCCc1c[nH]c2ccc(O)cc12 | ||
| Standard InChIKey | MDIGAZPGKJFIAH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H12N2O.ClH/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10;/h1-2,5-6,12-13H,3-4,11H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous agonist at 5-HT receptors and endogenous substrate for 5-HT transporters. Neurotransmitter that has roles in regulation of mood, emesis, sexuality, sleep and appetite in vivo. Caged serotonin is also available. |

Serotonin HCl Dilution Calculator

Serotonin HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7019 mL | 23.5095 mL | 47.019 mL | 94.038 mL | 117.5475 mL |
| 5 mM | 0.9404 mL | 4.7019 mL | 9.4038 mL | 18.8076 mL | 23.5095 mL |
| 10 mM | 0.4702 mL | 2.3509 mL | 4.7019 mL | 9.4038 mL | 11.7547 mL |
| 50 mM | 0.094 mL | 0.4702 mL | 0.9404 mL | 1.8808 mL | 2.3509 mL |
| 100 mM | 0.047 mL | 0.2351 mL | 0.4702 mL | 0.9404 mL | 1.1755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Serotonin HCl is a monoamine neurotransmitter and Endogenous 5-HT receptor agonist.
- H-D-Trp-OH
Catalog No.:BCC3117
CAS No.:153-94-6
- 2-Aminofluorene
Catalog No.:BCC8549
CAS No.:153-78-6
- Rutin
Catalog No.:BCN1684
CAS No.:153-18-4
- Guajadial F
Catalog No.:BCN6437
CAS No.:1529775-08-3
- Guajadial E
Catalog No.:BCN7754
CAS No.:1529775-06-1
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Dehydro-alpha-lapachone
Catalog No.:BCN1683
CAS No.:15297-92-4
- Nolatrexed (AG-337)
Catalog No.:BCC6430
CAS No.:152946-68-4
- Ginkgolide M
Catalog No.:BCC8178
CAS No.:15291-78-8
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Precursor of cefcapene diisopropylanmine salt
Catalog No.:BCC9127
CAS No.:153012-37-4
- SR 140333
Catalog No.:BCC6098
CAS No.:153050-21-6
- Diclofenac Sodium
Catalog No.:BCC4439
CAS No.:15307-79-6
- Diclofenac
Catalog No.:BCC5249
CAS No.:15307-86-5
- N-Methyltaxol C
Catalog No.:BCN7343
CAS No.:153083-53-5
- Thiamphenicol
Catalog No.:BCC4736
CAS No.:15318-45-3
- Taxayunnansin A
Catalog No.:BCN1685
CAS No.:153229-31-3
- Cilomilast
Catalog No.:BCC2283
CAS No.:153259-65-5
- ML355
Catalog No.:BCC8060
CAS No.:1532593-30-8
- 4,4'-Bis(2-benzoxazolyl)stilbene
Catalog No.:BCC8656
CAS No.:1533-45-5
- DFB
Catalog No.:BCC7130
CAS No.:15332-10-2
- SCR7
Catalog No.:BCC3978
CAS No.:1533426-72-0
The fate of (-)1-(benzofuran-2-yl)-2-propylaminopentane . HCl, (-)-BPAP, in rats, a potent enhancer of the impulse-evoked release of catecholamines and serotonin in the brain.[Pubmed:12365195]
Eur J Drug Metab Pharmacokinet. 2002 Jul-Sep;27(3):157-61.
Our aim was to study the fate of (-)-1-(benzofuran-2-yl)-2-propylaminopentane .HCl [(-)-BPAP] in rats, using radio-labelled compound [(-)-BPAP-14C]. Radioactivity was measured by liquid scintillation technique and the tissue concentrations of radioactivity were calculated on the basis of the specific activity of (-)-BPAP-14C and the values are given in ngeq./g. Radioactivity was well absorbed after i.p., s.c. and oral treatment and Cmax has been reached at 30 to 60 min following drug administration. A second peak, detected at 4 hours, indicated enterohepatic circulation of the substance. The highest tissue levels of radioactivity were reached at 30 min following s.c. treatment. The time-related changes of radioactivity were measured in nine selected brain regions after s.c. administration of (-)-BPAP-14C. A similar distribution profile was observed in the brain regions with a peak level at 30 min. Radioactivity is preferentially eliminated through the urine, the secondary route of excretion was the stool. More than 90% of the substance was recovered in the excreta during 72 hours. The t1/2 beta was found to be 5.5 to 5.8 hours. (-)-BPAP was well absorbed and penetrated the brain. Its elimination was fast and enterohepatic circulation was observed in rats.
In vivo effects of the putative cognitive enhancer KA-672.HCl in comparison with 8-hydroxy-2-(di-N-propylamino) tetralin and haloperidol on dopamine, 3,4-dihydroxyphenylacetic acid, serotonin and 5-hydroxyindoleacetic acid levels in striatal and cortical brain regions.[Pubmed:10800755]
Prog Neuropsychopharmacol Biol Psychiatry. 2000 Feb;24(2):337-48.
1. KA-672.HCl (7-methoxy-6-[3-[4-(2-methoxyphenyl)piperazin-1-yl]propoxy]-3,4-di methyl-2H-1-benzopyran-2-one hydrochloride), designed as a cognitive enhancer, has been investigated through behavioural and binding studies. However, little is known about its biochemical effects on the dopaminergic and serotoninergic system in vivo. 2. In the present study the authors investigated the effects of KA-672.HCl (0.1 mg/kg and 1 mg/kg), 8-hydroxy-2-(di-N-propylamino)tetralin (8-OH-DPAT) (1 mg/kg), haloperidol (0.1 mg/kg) and a mixture of haloperidol and 8-OH-DPAT on dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT) and 5-hydroxyindolacetic acid (5-HIAA) levels, in striatum and cerebral cortex of rats. 3. Male Wistar rats received an intraperitoneal injection of the drugs or vehicle 1 hour before striatal and cortical brain tissues were dissected out for neurochemical analysis. 4. KA-672.HCl, 8-OH-DPAT and haloperidol significantly reduced striatal DA levels, whereas only KA-672.HCl significantly reduced cortical DA levels. 8-OH-DPAT and haloperidol induced a significant increase in cortical DOPAC levels but only haloperidol significantly elevated the striatal DOPAC content. In contrast, only the higher dose of KA-672.HCl elevated striatal DOPAC levels. Furthermore, KA-672.HCl significantly reduced striatal 5-HT levels and slightly elevated striatal 5-HIAA concentrations. 8-OH-DPAT significantly decreased striatal 5-HIAA levels. All substances were able to enhance the cortical and striatal DA turnover. 5. The cortical and striatal 5-HT turnover was significantly decreased following 8-OH-DPAT treatment and significantly increased in the striatum after haloperidol and KA-672.HCl treatment. 6. The data suggest that KA-672.HCl possesses D2 antagonistic as well as 5-HT1A agonistic properties. However, additional mechanisms of actions by interaction with other neurotransmitter systems such as acetylcholine, excitatory or inhibitory amino acids need to be determined.
Stereochemistry of serotonin receptor ligands from crystallographic data. Crystal structures of NAN-190.HBr, 1-phenylbiguanide, MDL 72222 and mianserin.HCl and selectivity criteria towards 5-HT1, 5-HT2, and 5-HT3 receptor subtypes.[Pubmed:8767764]
Acta Crystallogr B. 1996 Jun 1;52 ( Pt 3):509-18.
The crystal and molecular structures of the following serotoninergic drugs have been determined: (1) 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine hydrobromide hemihydrate (NAN-190.HBr), C23H28N3O3+.Br-.1/2H2O, M(r) = 483.42, monoclinic, C2/c, a = 21.916 (4), b = 15.207 (2), c = 14.052 (2) A, beta = 101.56 (1) degree, V = 4588 (1) A3, Z = 8, Dx = 1.40 Mgm-3, lambda (Mo K alpha) = 0.71069 A, mu = 1.823 mm-1, F(000) = 2008, T = 295 K, R = 0.035 for 2617 observed reflections; (2) N-phenylimidocarbonimidic diamide (1-phenylbiguanide), C8H11N5, M(r) = 177.21, monoclinic, P2(1)/c, a = 9.781 (2), b = 35.040(5), c = 11.000 (2) A, beta = 97.72(1) degree, V = 3736(1)A3, Z = 16, Dx = 1.26 Mg m-3, lambda (Mo K alpha) = 0.71069 A, mu = 0.084 mm-1, F(000) = 1504, T = 295 K, R = 0.070 for 3407 observed reflections; (3) 8-methyl-8-azabicyclo[3.2.1]oct-3yl 3,5-dicholorobenzoate (MDL 72222), C15H17Cl2NO2, M(r) = 314.21, triclinic, P1, alpha = 8.480 (3), b = 9.840 (3), c = 10.158 (4) A, alpha = 90.04 (3), beta = 111.77 (3), gamma = 105.07(3) degrees, V = 755.6(5) A3, Z = 2, Dx = 1.38 Mg m-3, lambda(Mo K alpha) = 0.71069 A, mu = 0.430 mm-1, F(000) = 328, T = 295 K, R = 0.070 for 1685 observed reflections; (4) 1, 2, 3, 4, 10, 14b-hexahydro-2-methyldibenzo[c.f]pyrizino[1, 2-alpha]azepine hydrochloride (mianserin. HCl), C18H21N2+. Cl-, M(r) = 300.83, monoclinic, P2(1)/a, a = 9.014 (2), b = 14.917 (2), c = 12.412 (2) A, beta = 108.84 (1) degree, V = 1579.5 (5) A3, Z = 4, Dx = 1.26 Mg m-3, lambda(Mo K alpha) = 0.71069 A, mu = 0.237 mm-1, F(000) = 640, T = 295 K, R = 0.063 for 1493 observed reflections. A systematic structural analysis of the present compounds and others known to interact with the 5-HT1, 5-HT2 and 5-HT3 receptors allows to identify their similarities with the endogenous ligand serotonin (5-HT) and the stereochemical differences which determine selectivity for the various receptor subtypes. The pharmacophoric feature for 5-HT receptor binding is identified in a constant-length vector linking an aromatic ring with a protonated nitrogen, while specific affinities for receptorial subtypes and the nature of the effect appear to be modulated by the dimensions of the substituents at nitrogen.
A review of central 5-HT receptors and their function.[Pubmed:10462127]
Neuropharmacology. 1999 Aug;38(8):1083-152.
It is now nearly 5 years since the last of the currently recognised 5-HT receptors was identified in terms of its cDNA sequence. Over this period, much effort has been directed towards understanding the function attributable to individual 5-HT receptors in the brain. This has been helped, in part, by the synthesis of a number of compounds that selectively interact with individual 5-HT receptor subtypes--although some 5-HT receptors still lack any selective ligands (e.g. 5-ht1E, 5-ht5A and 5-ht5B receptors). The present review provides background information for each 5-HT receptor subtype and subsequently reviews in more detail the functional responses attributed to each receptor in the brain. Clearly this latter area has moved forward in recent years and this progression is likely to continue given the level of interest associated with the actions of 5-HT. This interest is stimulated by the belief that pharmacological manipulation of the central 5-HT system will have therapeutic potential. In support of which, a number of 5-HT receptor ligands are currently utilised, or are in clinical development, to reduce the symptoms of CNS dysfunction.


