SCH 563705CXCR2/CXCR1 antagonist,potent CAS# 473728-58-4 |
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- Reparixin L-lysine salt
Catalog No.:BCC1886
CAS No.:266359-93-7
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 473728-58-4 | SDF | Download SDF |
| PubChem ID | 10310100 | Appearance | Powder |
| Formula | C23H27N3O5 | M.Wt | 425.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 30 mg/mL (70.51 mM) *"≥" means soluble, but saturation unknown. | ||
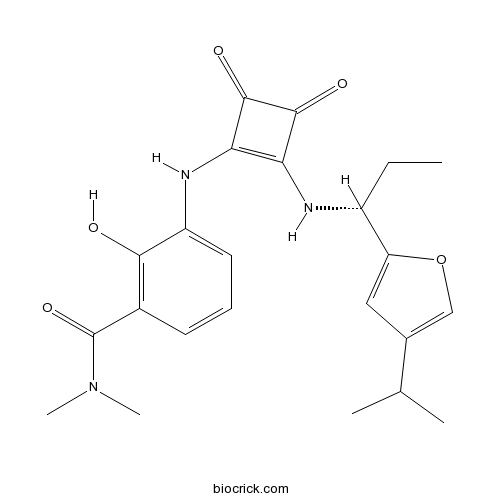
| Chemical Name | 3-[[3,4-dioxo-2-[[(1R)-1-(4-propan-2-ylfuran-2-yl)propyl]amino]cyclobuten-1-yl]amino]-2-hydroxy-N,N-dimethylbenzamide | ||
| SMILES | CCC(C1=CC(=CO1)C(C)C)NC2=C(C(=O)C2=O)NC3=CC=CC(=C3O)C(=O)N(C)C | ||
| Standard InChIKey | DGKQQEVYYPCMNE-OAHLLOKOSA-N | ||
| Standard InChI | InChI=1S/C23H27N3O5/c1-6-15(17-10-13(11-31-17)12(2)3)24-18-19(22(29)21(18)28)25-16-9-7-8-14(20(16)27)23(30)26(4)5/h7-12,15,24-25,27H,6H2,1-5H3/t15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SCH 563705 is a potent dual CXCR2(IC50= 1.3 nM)/CXCR1(IC50= 7.3 nM) antagonist.
IC50 value: 7.3 nM (CXCR1); 1.3 nM (CXCR2) [1]
Target: CXCR1; CXCR2
in vitro: SCH 563705 has emerged as the most potent CXCR2–CXCR1 dual antagonist to date. It has been evaluated further in a wide range of in vitro and in vivo studies. In the chemotaxis assays, it demonstrated potent inhibition against both Gro-α and IL-8 induced human neutrophil migration (chemotaxis IC50 = 0.5 nM, against 30 nM of Gro-α; chemotaxis IC50 = 37 nM, against 3 nM of IL-8) [1]. SCH563705 selectively reduced the peripheral blood neutrophil frequency, and caused an elevation in the CXCR2 ligand CXCL1 [2].
in vivo: SCH 563705 achieved an ED50 of 0.9 mg/kg in the rat LPS (lipopolysaccharide) challenged model and an ED50 of 1 mg/kg in the mouse cigarette smoke induced neutrophilic inflammatory model [1]. References: | |||||

SCH 563705 Dilution Calculator

SCH 563705 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3503 mL | 11.7514 mL | 23.5029 mL | 47.0057 mL | 58.7572 mL |
| 5 mM | 0.4701 mL | 2.3503 mL | 4.7006 mL | 9.4011 mL | 11.7514 mL |
| 10 mM | 0.235 mL | 1.1751 mL | 2.3503 mL | 4.7006 mL | 5.8757 mL |
| 50 mM | 0.047 mL | 0.235 mL | 0.4701 mL | 0.9401 mL | 1.1751 mL |
| 100 mM | 0.0235 mL | 0.1175 mL | 0.235 mL | 0.4701 mL | 0.5876 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SCH 563705, a potent CXCR2 and CXCR1 antagonist, has therapeutic potential for targeting CXCR2/CXCR1 in human arthritides.
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- AMG 487
Catalog No.:BCC5140
CAS No.:473719-41-4
- Boc-Trp(For)-OH
Catalog No.:BCC3456
CAS No.:47355-10-2
- Carpinontriol B
Catalog No.:BCN8113
CAS No.:473451-73-9
- Spegatrine
Catalog No.:BCN4068
CAS No.:47326-53-4
- Betulin
Catalog No.:BCN5528
CAS No.:473-98-3
- Tolbutamide Sodium
Catalog No.:BCC5632
CAS No.:473-41-6
- beta-Eudesmol
Catalog No.:BCN6294
CAS No.:473-15-4
- alpha-Cyperone
Catalog No.:BCN1193
CAS No.:473-08-5
- SB 366791
Catalog No.:BCC7128
CAS No.:472981-92-3
- 8(14),15-Isopimaradien-3-ol
Catalog No.:BCN5526
CAS No.:4728-30-7
- 1-Benzyl-4-hydroxypiperidine
Catalog No.:BCC8459
CAS No.:4727-72-4
- Boc-Tyr(tBu)-OH
Catalog No.:BCC3462
CAS No.:47375-34-8
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Forskolin G
Catalog No.:BCN5527
CAS No.:473981-11-2
- Brazilin
Catalog No.:BCN5529
CAS No.:474-07-7
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- Citrostadienol
Catalog No.:BCN7357
CAS No.:474-40-8
- Reserpin N-oxide
Catalog No.:BCN3493
CAS No.:474-48-6
- Daucosterol
Catalog No.:BCN5531
CAS No.:474-58-8
- Campestanol
Catalog No.:BCN3890
CAS No.:474-60-2
- Campesterol
Catalog No.:BCN3181
CAS No.:474-62-4
- Brassicasterol
Catalog No.:BCN2613
CAS No.:474-67-9
- CAL-130 Racemate
Catalog No.:BCC1442
CAS No.:474012-90-3
SEM studies on acanthocephalan parasite, Echinorhynchus veli infecting the fish Synaptura orientalis (Bl & Sch, 1801).[Pubmed:28316390]
J Parasit Dis. 2017 Mar;41(1):71-75.
Echinorhynchus veli (George and Nadakal, 1978), an acanthocephalid worm infesting the estuarine flat fish, Synaptura orientalis, was collected from the Veli lake, Kerala. The parasite was recovered from the intestine of the host fish. The detailed surface morphology was studied with the help of scanning electron microscope. The study revealed a cylindrical, medially swollen proboscis with a flat apex, backward directed hooks, each with smooth surface, broad base, pointed tip and an epidermal elevation at the point of insertion. A pair of sensory pits was seen at the base of the proboscis. The neck was well developed with densely packed epidermal micropores. Paired sensory pits were seen at the base of the neck and a collar between it and the trunk. The epidermis of the trunk has microtriches and micropores. The female genital pore was circular, and terminal in an elevated orifice. In male, the copulatory bursa was directed ventrally, with well-defined rim and several sensory papillae.
Structural Determination of (-)-SCH 64874 and Hirsutellomycin by Semisynthesis.[Pubmed:27966974]
J Org Chem. 2017 Jan 6;82(1):353-371.
The structure of a C2-symmetric epidithiodiketopiperazine alkaloid, SCH 64874, was determined by semisynthesis. The relative stereochemistry of the beta-hydroxy carboxylic acid chain having three chiral centers was determined by comparison of the NMR data of the four possible diastereomeric beta-hydroxy carboxylic acid fragments with those of SCH 64874. Condensation of the (-)-deacetylaranotin core with two enantiomeric beta-hydroxy carboxylic acids revealed the relative stereochemistry of SCH 64874. The relative stereochemistry of the beta-keto carboxylic acid chain of the analogous alkaloid hirsutellomycin was determined in a stepwise manner. The C4'-C6' syn relationships were predicted by comparing the NMR data of the corresponding ester fragments with that of hirsutellomycin. The relative stereochemistry of the whole molecule, including the epimerizable C2' stereocenter, was determined by introduction of four possible side chains into the bisdethiodi(methylthio)deacetylaranotin core. We found that the stereochemistry of C2' converged with that of the thermodynamically stable form influenced by the core structure.
SCH 79797, a selective PAR1 antagonist, protects against ischemia/reperfusion-induced arrhythmias in the rat hearts.[Pubmed:27906419]
Eur Rev Med Pharmacol Sci. 2016 Nov;20(22):4796-4800.
OBJECTIVE: Thrombin is implicated in the genesis of arrhythmias and activation of thrombin receptors exacerbated ventricular arrhythmias following coronary artery ligation. The present study was designed to investigate the possible protective effect of the protease-activated receptor-1 antagonist, SCH79797 against ischemia and reperfusion arrhythmias in the rat heart. MATERIALS AND METHODS: Healthy male Wistar rats (250-350 g) were anesthetized with urethane. Coronary artery ligation was performed for 5 minutes followed by 10 minutes reperfusion. Rhythm disturbances were monitored throughout the ischemia and reperfusion periods. Drugs injected were SCH79797 dihydrochloride (6.25, 12.5, 25 and 100 microg/kg), glibenclamide (5 mg/kg) and N-nitro L-arginine methyl-ester hydrochloride (25 mg/kg). The control group was injected with dimethylsulfoxide (0.1 ml). RESULTS: SCH79797 dihydrochloride reduced the number of premature contraction, prevalence and duration of ventricular tachycardia, prevalence and duration of ventricular fibrillation during both the ischemic and reperfusion periods in a dose-dependent manner. There is a trend for N-nitro L-arginine methyl-ester hydrochloride to increase all parameters of arrhythmias in SCH79797 dihydrochloride (25 microg/kg) treated rats, but glibenclamide (5 mg/kg) significantly (p < 0.05) increased these parameters. CONCLUSIONS: SCH79797 dihydrochloride induced an antiarrhythmic effect in the anesthetized rat. This protective effect could possibly be mediated by activation of nitric oxide synthase and/or of ATP-sensitive potassium channels.
Design and synthesis of water soluble beta-aminosulfone analogues of SCH 900229 as gamma-secretase inhibitors.[Pubmed:27836402]
Bioorg Med Chem Lett. 2016 Dec 1;26(23):5836-5841.
In this paper we describe our strategy to improve the aqueous solubility of SCH 900229, a potent PS1-selective gamma-secretase inhibitor for the treatment of Alzheimer's disease. Incorporation of ionizable amino groups into the side chain terminal generates water soluble beta-aminosulfone analogues of SCH 900229 that maintain robust in vitro potency and in vivo efficacy.


