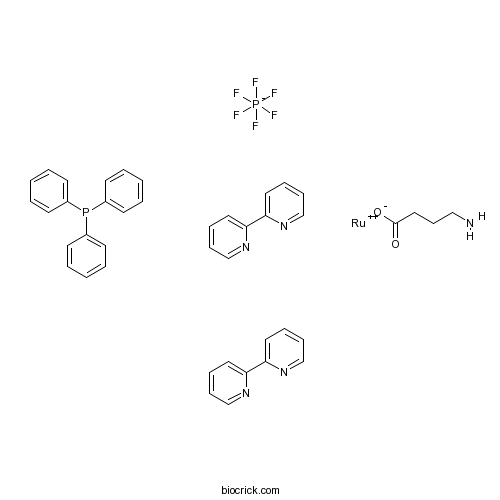
RuBi-GABACaged GABA; excitable by visible wavelength CAS# 1028141-88-9 |
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1028141-88-9 | SDF | Download SDF |
| PubChem ID | 129316180 | Appearance | Powder |
| Formula | C42H39F6N5O2P2Ru | M.Wt | 922.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in sterile water | ||
| Chemical Name | 4-aminobutanoate;2-pyridin-2-ylpyridine;ruthenium(2+);triphenylphosphane;hexafluorophosphate | ||
| SMILES | C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=NC(=C1)C2=CC=CC=N2.C1=CC=NC(=C1)C2=CC=CC=N2.C(CC(=O)[O-])CN.F[P-](F)(F)(F)(F)F.[Ru+2] | ||
| Standard InChIKey | SWFWLLYVSYURHZ-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C18H15P.2C10H8N2.C4H9NO2.F6P.Ru/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;2*1-3-7-11-9(5-1)10-6-2-4-8-12-10;5-3-1-2-4(6)7;1-7(2,3,4,5)6;/h1-15H;2*1-8H;1-3,5H2,(H,6,7);;/q;;;;-1;+2/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ruthenium-bipyridine-triphenylphosphine caged GABA that is excited by visible wavelengths. Provides greater tissue penetration, less phototoxicity, faster photorelease kinetics and better spatial resolution than UV light-sensitive caged compounds. Produces GABA receptor-mediated currents in pyramidal neurons in vitro and displays no effect on endogenous GABAergic or glutamatergic transmission at concentrations effective for uncaging. |

RuBi-GABA Dilution Calculator

RuBi-GABA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0837 mL | 5.4183 mL | 10.8366 mL | 21.6732 mL | 27.0915 mL |
| 5 mM | 0.2167 mL | 1.0837 mL | 2.1673 mL | 4.3346 mL | 5.4183 mL |
| 10 mM | 0.1084 mL | 0.5418 mL | 1.0837 mL | 2.1673 mL | 2.7091 mL |
| 50 mM | 0.0217 mL | 0.1084 mL | 0.2167 mL | 0.4335 mL | 0.5418 mL |
| 100 mM | 0.0108 mL | 0.0542 mL | 0.1084 mL | 0.2167 mL | 0.2709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
- Lycopsamine
Catalog No.:BCN1999
CAS No.:10285-07-1
- 17-Hydroxy sprengerinin C
Catalog No.:BCN2755
CAS No.:1029017-75-1
Recording of Neural Activity with Modulation of Photolysis of Caged Compounds using Microelectrode Arrays in Rats with Seizures.[Pubmed:30794501]
IEEE Trans Biomed Eng. 2019 Feb 20.
OBJECTIVE: In this work, a new method was established to monitor multichannel neural activity with microelectrode arrays (MEAs) under modulation of caged compounds in a rat model of seizures. METHODS: The 16-channel MEAs were fabricated and implanted into the hippocampus of normal rats and epileptic rats for neural spike and local field potential (LFP) recording. Using optical fibers with drug delivery tubing, two different caged compounds (ruthenium-bipyridine-trimethylphosphine glutamate (RuBi-Glu) and ruthenium-bipyridine-trimethylphosphine gamma aminobutyric acid (RuBi-GABA)) were applied, and blue light (465 nm) was used to modulate neural activity. RESULTS: In normal rats, RuBi-Glu excited neural activity and RuBi-GABA inhibited neural activity. The amplitude of spikes increased 26% from 154 muV to 194 muV with RuBi-Glu modulation. During RuBi-GABA modulation, spikes recovered to 142 muV. The firing rate increased from 1.4 Hz to 4.5 Hz with RuBi-Glu modulation and decreased to 0.8 Hz after RuBi-GABA modulation. The power of LFPs increased from 566 muW to 1128 muW with RuBi-Glu modulation and recovered to 710 muW with RuBi-GABA modulation. In epileptic rats, the neural activity during seizures was significantly inhibited by RuBi-GABA modulation. The amplitude of spikes was 242 muV during seizures and decreased to 112 muV with RuBi-GABA modulation. The firing rate decreased from 20.29 Hz to 1.33 Hz with RuBi-GABA modulation. CONCLUSION: Using MEAs, the modulation of neural activity with caged compound photolysis was observed with high temporal-spatial resolution in normal and epileptic rats. SIGNIFICANCE: This new method is important for monitoring neural activity with photo-switchable modulation.
Photolysis of Caged-GABA Rapidly Terminates Seizures In Vivo: Concentration and Light Intensity Dependence.[Pubmed:28572790]
Front Neurol. 2017 May 18;8:215.
The therapy of focal epilepsy remains unsatisfactory for as many as 25% of patients. The photolysis of caged-gamma-aminobutyric acid (caged-GABA) represents a novel and alternative option for the treatment of intractable epilepsy. Our previous experimental results have demonstrated that the use of blue light produced by light-emitting diode to uncage ruthenium-bipyridine-triphenylphosphine-c-GABA (RuBi-GABA) can rapidly terminate paroxysmal seizure activity both in vitro and in vivo. However, the optimal concentration of RuBi-GABA, and the intensity of illumination to abort seizures, remains unknown. The aim of this study was to explore the optimal anti-seizure effects of RuBi-GABA by using implantable fibers to introduce blue light into the neocortex of a 4-aminopyridine-induced acute seizure model in rats. We then investigated the effects of different combinations of RuBi-GABA concentrations and light intensity upon seizure. Our results show that the anti-seizure effect of RuBi-GABA has obvious concentration and light intensity dependence. This is the first example of using an implantable device for the photolysis of RuBi-GABA in the therapy of neocortical seizure, and an optimal combination of RuBi-GABA concentration and light intensity was explored. These results provide important experimental data for future clinical translational studies.
Biphasic modulation of parallel fibre synaptic transmission by co-activation of presynaptic GABAA and GABAB receptors in mice.[Pubmed:27061582]
J Physiol. 2016 Jul 1;594(13):3651-66.
KEY POINTS: Many excitatory synapses co-express presynaptic GABAA and GABAB receptors, despite their opposing actions on synaptic transmission. It is still unclear how co-activation of these receptors modulates synapse function. We measured presynaptic GABA receptor function at parallel fibre synapses onto stellate cells in the cerebellum using whole-cell patch-clamp recording and photolytic uncaging of RuBi-GABA. Activation of presynaptic GABA receptors results in a transient ( approximately 100 ms) enhancement of synaptic transmission (mediated by GABAA receptors) followed by a long lasting (>500 ms) inhibition of transmission (mediated by GABAB receptors). When activated just prior to high-frequency trains of stimulation, presynaptic GABAA and GABAB receptors work together to reduce short-term facilitation/enhance depression, altering the filtering properties of synaptic transmission. Inhibition of synaptic transmission by GABAB receptors is more sensitive to GABA than enhancement by GABAA receptors, suggesting GABAB receptors may be activated by ambient GABA or release from greater distances. ABSTRACT: GABAA and GABAB receptors are co-expressed at many presynaptic terminals in the central nervous system. Previous studies have shown that GABAA receptors typically enhance vesicle release while GABAB receptors inhibit release. However, it is not clear how the competing actions of these receptors modulate synaptic transmission when co-activated, as is likely in vivo. We investigated this question at parallel fibre synapses in the cerebellum, which co-express presynaptic GABAA and GABAB receptors. In acute slices from C57BL/6 mice, we find that co-activation of presynaptic GABA receptors by photolytic uncaging of RuBi-GABA has a biphasic effect on EPSC amplitudes recorded from stellate cells. Synchronous and asynchronous EPSCs evoked within approximately 100 ms of GABA uncaging were increased, while EPSCs evoked approximately 300-600 ms after GABA uncaging were reduced compared to interleaved control sweeps. We confirmed these effects are presynaptic by measuring the paired-pulse ratio, variance of EPSC amplitudes, and response probability. During trains of high-frequency stimulation GABAA and GABAB receptors work together (rather than oppose one another) to reduce short-term facilitation when GABA is uncaged just prior to the onset of stimulation. We also find that GABAB receptor-mediated inhibition can be elicited by lower GABA concentrations than GABAA receptor-mediated enhancement of EPSCs, suggesting GABAB receptors may be selectively activated by ambient GABA or release from more distance synapses. These data suggest that GABA, acting through both presynaptic GABAA and GABAB receptors, modulate the amplitude and short-term plasticity of excitatory synapses, a result not possible from activation of either receptor type alone.
Caged compounds for multichromic optical interrogation of neural systems.[Pubmed:25471355]
Eur J Neurosci. 2015 Jan;41(1):5-16.
Caged compounds are widely used by neurophysiologists to study many aspects of cellular signaling in glia and neurons. Biologically inert before irradiation, they can be loaded into cells via patch pipette or topically applied in situ to a defined concentration; photolysis releases the caged compound in a very rapid and spatially defined way. As caged compounds are exogenous optical probes, they include not only natural products such neurotransmitters, calcium and IP3 but non-natural products such as fluorophores, drugs and antibodies. In this Technical Spotlight we provide a short introduction to the uncaging technique by discussing the nitroaromatic caging chromophores most widely used in such experiments [e.g. alpha-carboxy-ortho-nitrobenyl (CNB), dimethoxynitrobenzyl (DMNB), 4-methoxy-7-nitroindolinyl (MNI) and 4-carboxymethoxy-7-nitroindolinyl (CDNI)]. We show that recently developed caging chromophores [rutheniumbipyridial (RuBi) and 7-diethylaminocoumarin (DEAC)450] that are photolyzed with blue light (~ 430-480 nm range) can be combined with traditional nitroaromatic caged compounds to enable two-color optical probing of neuronal function. For example, one-photon uncaging of either RuBi-GABA or DEAC450-GABA with a 473-nm laser is facile, and can block nonlinear currents (dendritic spikes or action potentials) evoked by two-photon uncaging of CDNI-Glu at 720 nm. We also show that two-photon uncaging of DEAC450-Glu and CDNI-GABA at 900 and 720 nm, respectively, can be used to fire and block action potentials. Our experiments illustrate that recently developed chromophores have taken uncaging out of the 'monochrome era', in which it has existed since 1978, so as to enable multichromic interrogation of neuronal function with single-synapse precision.
Activation of axonal receptors by GABA spillover increases somatic firing.[Pubmed:24155298]
J Neurosci. 2013 Oct 23;33(43):16924-9.
Axons can be depolarized by ionotropic receptors and transmit subthreshold depolarizations to the soma by passive electrical spread. This raises the possibility that axons and axonal receptors can participate in integration and firing in neurons. Previously, we have shown that exogenous GABA depolarizes cerebellar granule cell axons through local activation of GABA(A) receptors (GABA(A)Rs) and the soma through electrotonic spread of the axonal potential resulting in increased firing. We show here that excitability of granule cells is also increased by release of endogenous GABA from molecular layer interneurons (MLIs) and spillover activation of parallel fiber GABA(A)Rs in mice and rats. Changes in granule cell excitability were assessed by excitability testing after activation of MLIs with channelrhodopsin or electrical stimulation in the molecular layer. In granule cells lacking an axon, excitability was not changed, suggesting that axonal receptors are required. To determine the distance over which subthreshold potentials may spread, we estimated the effective axonal electrical length constant (520 mum) by excitability testing and focal uncaging of RuBi-GABA on the axon at varying distances from the soma. These data suggest that GABA(A)R-mediated axonal potentials can participate in integration and firing of cerebellar granule cells.
Cell-attached recordings of responses evoked by photorelease of GABA in the immature cortical neurons.[Pubmed:23754981]
Front Cell Neurosci. 2013 May 31;7:83.
We present a novel non-invasive technique to measure the polarity of GABAergic responses based on cell-attached recordings of currents activated by laser-uncaging of GABA. For these recordings, a patch pipette was filled with a solution containing RuBi-GABA, and GABA was released from this complex by a laser beam conducted to the tip of the patch pipette via an optic fiber. In cell-attached recordings from neocortical and hippocampal neurons in postnatal days P2-5 rat brain slices in vitro, we found that laser-uncaging of GABA activates integral cell-attached currents mediated by tens of GABA(A) channels. The initial response was inwardly directed, indicating a depolarizing response to GABA. The direction of the initial response was dependent on the pipette potential and analysis of its slope-voltage relationships revealed a depolarizing driving force of +11 mV for the currents through GABA channels. Initial depolarizing responses to GABA uncaging were inverted to hyperpolarizing in the presence of the NKCC1 blocker bumetanide. Current-voltage relationships of the currents evoked by RuBi-GABA uncaging using voltage-ramps at the peak of responses not only revealed a bumetanide-sensitive depolarizing reversal potential of the GABA(A) receptor mediated responses, but also showed a strong voltage-dependent hysteresis. Upon desensitization of the uncaged-GABA response, current-voltage relationships of the currents through single GABA(A) channels revealed depolarizing responses with the driving force values similar to those obtained for the initial response. Thus, cell-attached recordings of the responses evoked by local intrapipette GABA uncaging are suitable to assess the polarity of the GABA(A)-Rs mediated signals in small cell compartments.
Optical control of focal epilepsy in vivo with caged gamma-aminobutyric acid.[Pubmed:22275253]
Ann Neurol. 2012 Jan;71(1):68-75.
OBJECTIVE: There is enormous clinical potential in exploiting the spatial and temporal resolution of optical techniques to modulate pathophysiological neuronal activity, especially intractable focal epilepsy. We have recently utilized a new ruthenium-based caged compound, ruthenium-bipyridine-triphenylphosphine-gamma-aminobutyric acid (RuBi-GABA), which releases GABA when exposed to blue light, to rapidly terminate paroxysmal activity in vitro and in vivo. METHODS: The convulsant 4-aminopyridine was used to induce interictal activity and seizures in rat neocortical slices and anesthetized rats. We examined the effect of blue light, generated by a small, light-emitting diode (LED), on the frequency and duration of ictal activity in the presence and absence of RuBi-GABA. RESULTS: Neither blue light alone, nor low concentrations of RuBi-GABA, affected interictal activity or baseline electrical activity in neocortical slices. However, brief, blue illumination of RuBi-GABA, using our LED, dramatically reduced extracellular spikes and bursts. More impressively, illumination of locally applied RuBi-GABA rapidly terminated in vivo seizures induced by topical application of 4-aminopyridine. The RuBi-GABA effect was blocked by the GABA(A) antagonist picrotoxin, but not duplicated by direct application of GABA. INTERPRETATION: This is the first example of optical control of in vivo epilepsy, proving that there is sufficient cortical light penetration from an LED and diffusion of caged GABA to quickly terminate intense focal seizures. We are aware that many obstacles need to be overcome before this technique can be translated to patients, but at the moment, this represents a feasible method for harnessing optical techniques to fabricate an implantable device for the therapy of neocortical epilepsy.
Spatial distributions of GABA receptors and local inhibition of Ca2+ transients studied with GABA uncaging in the dendrites of CA1 pyramidal neurons.[Pubmed:21799926]
PLoS One. 2011;6(7):e22652.
GABA (gamma-amino-butylic acid)-mediated inhibition in the dendrites of CA1 pyramidal neurons was characterized by two-photon uncaging of a caged-GABA compound, BCMACM-GABA, and one-photon uncaging of RuBi-GABA in rat hippocampal slice preparations. Although we found that GABA(A)-mediated currents were diffusely distributed along the dendrites, currents elicited at the branch points of the apical dendritic trunk were approximately two times larger than those elsewhere in the dendrite. We examined the inhibitory action of the GABA-induced currents on Ca(2+) transients evoked with a single back-propagating action potential (bAP) in oblique dendrites. We found that GABA uncaging selectively inhibited the Ca(2+) transients in the region adjacent (<20 microm) to the uncaging site, and that GABA uncaging was effective only within a short period after bAP (<20 ms). The strength of inhibition was linearly related to the amplitudes of the GABA currents, suggesting that the currents inhibited a sustained, subthreshold after-depolarization without preventing propagation of bAP. GABA uncaging at the dendritic branch points inhibited Ca(2+) transients farther into dendritic branches (>20 microm). Our data indicate that GABA inhibition results in spatially confined inhibition of Ca(2+) transients shortly after bAP, and suggest that this effect is particularly potent at the dendritic branch points where GABA receptors cluster.
Photorelease of GABA with Visible Light Using an Inorganic Caging Group.[Pubmed:18946542]
Front Neural Circuits. 2008 Aug 13;2:2.
We describe the selective photorelease of gamma-amino butyric acid (GABA) with a novel caged-GABA compound that uses a ruthenium complex as photosensor. This compound ("RuBi-GABA") can be excited with visible wavelengths, providing greater tissue penetration, less photo-toxicity, and faster photorelease kinetics than currently used UV light-sensitive caged compounds. Using pyramidal neurons from neocortical brain slices, we show that RuBi-GABA uncaging induces GABA-A receptor-mediated responses, has no detectable side effects on endogenous GABAergic and glutamatergic receptors and generates responses with kinetics and spatial resolution comparable to the best caged GABA compounds presently available. Finally, we illustrate two potential applications of RuBi-GABA uncaging: GABA receptor mapping, and optical silencing of neuronal firing.
A new strategy for neurochemical photodelivery: metal-ligand heterolytic cleavage.[Pubmed:12537482]
J Am Chem Soc. 2003 Jan 29;125(4):882-3.
A new strategy to build caged-compounds is presented. The approach is based on heterolytic photocleavage of a metal-ligand bond in a coordination compound. A ruthenium polypyridine complex, containing the neurocompound 4-amino pyridine (4AP) is used as the core of the phototrigger. The biomolecule is released by irradiation with visible light (>480 nm). The liberated 4AP promotes the activation of a leech neuron by means of blocking its K+ channels. The syntesis, characterization, and the inherent advantages of this method are discussed.


