4-Acetyl RamelteonCAS# 1346598-94-4 |
Quality Control & MSDS
Number of papers citing our products

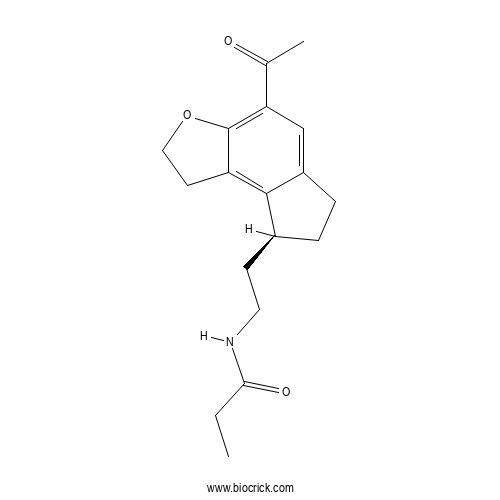
Chemical structure

3D structure
| Cas No. | 1346598-94-4 | SDF | Download SDF |
| PubChem ID | 71312980 | Appearance | Powder |
| Formula | C18H23NO3 | M.Wt | 301.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N-[2-[(8S)-4-acetyl-2,6,7,8-tetrahydro-1H-cyclopenta[e][1]benzofuran-8-yl]ethyl]propanamide | ||
| SMILES | CCC(=O)NCCC1CCC2=CC(=C3C(=C12)CCO3)C(=O)C | ||
| Standard InChIKey | TYKIXTOHVJPYMI-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C18H23NO3/c1-3-16(21)19-8-6-12-4-5-13-10-15(11(2)20)18-14(17(12)13)7-9-22-18/h10,12H,3-9H2,1-2H3,(H,19,21)/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Acetyl Ramelteon Dilution Calculator

4-Acetyl Ramelteon Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3179 mL | 16.5893 mL | 33.1785 mL | 66.357 mL | 82.9463 mL |
| 5 mM | 0.6636 mL | 3.3179 mL | 6.6357 mL | 13.2714 mL | 16.5893 mL |
| 10 mM | 0.3318 mL | 1.6589 mL | 3.3179 mL | 6.6357 mL | 8.2946 mL |
| 50 mM | 0.0664 mL | 0.3318 mL | 0.6636 mL | 1.3271 mL | 1.6589 mL |
| 100 mM | 0.0332 mL | 0.1659 mL | 0.3318 mL | 0.6636 mL | 0.8295 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- 25S-Inokosterone
Catalog No.:BCN3873
CAS No.:19595-18-7
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
A Review of Suvorexant, Doxepin, Ramelteon, and Tasimelteon for the Treatment of Insomnia in Geriatric Patients.[Pubmed:28270270]
Consult Pharm. 2017 Mar 1;32(3):156-160.
Geriatric patients often experience insomnia because of physiological and neurological changes that occur during the aging process. Use of benzodiazepines, nonbenzodiazepine hypnotics, and diphenhydramine for the treatment of insomnia pose an increased risk of cognitive impairment, falls, and fractures in this patient population. Therapeutic alternatives approved by the Food and Drug Administration include suvorexant, doxepin, ramelteon, and tasimelteon, which have shown efficacy and safety in various studies. This paper explores and outlines the available safety and efficacy data associated with these medications and reviews their potential place in therapy in treating insomnia in the geriatric population.
The Relief Effects of Ramelteon on Refractory Chronic Migraine: A Case Report.[Pubmed:27776398]
Clin Psychopharmacol Neurosci. 2016 Nov 30;14(4):405-406.
The selective melatonin receptor agonism effect of ramelteon is useful for insomnia. Here we wanted to present a refractory chronic migraine case, who had significant improvements in migraine after using ramelteon. The possible mechanism for the ramelteon in the migraine relief might be related to melatonin effects.
The Effects of Ramelteon on Glucose Metabolism and Sleep Quality in Type 2 Diabetic Patients With Insomnia: A Pilot Prospective Randomized Controlled Trial.[Pubmed:27829954]
J Clin Med Res. 2016 Dec;8(12):878-887.
BACKGROUND: Insomnia is associated with the onset and development of diabetes. Melatonin affects sleep quality and glucose metabolism in diabetic patients with insomnia. We administered ramelteon, an agonist of melatonin, to type 2 diabetic patients and investigated its effects on glucose metabolism and insomnia. METHODS: This multicenter, prospective, randomized, and observational pilot study was performed between April 2014 and April 2015 at three institutes in Japan. Patients were prescribed ramelteon 8 mg/day for 3 months (first period). And patients were divided at random into the continuation group that continued taking ramelteon and the discontinuation group that discontinued taking ramelteon for 3 additional months (second period). The primary endpoint was change in glycated hemoglobin (HbA1c) level. Secondary endpoints were changes in global Pittsburgh sleep questionnaire index (PSQI) score and other glucose metabolism makers. RESULTS: We enrolled 42 patients, and 32 patients completed the first period. Their mean HbA1c was 6.7%, and global PSQI score was 8.1 on average. HbA1c level did not change but global PSQI score improved from 8.1 to 7.2 by ramelteon (P = 0.030). Thirty-one patients completed the second period. HbA1c level did not change in the continuation group, but it increased from 6.7% to 6.9% (P = 0.003) in the discontinuation group. Global PSQI score did not change in each group. There was no rebound insomnia. CONCLUSION: Treatment with ramelteon did not change the HbA1c level but improved sleep quality in type 2 diabetic patients with insomnia. Discontinuation of ramelteon slightly increased the HbA1c level and did not worsen sleep quality.


