RU 58668Pure antiestrogen CAS# 151555-47-4 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
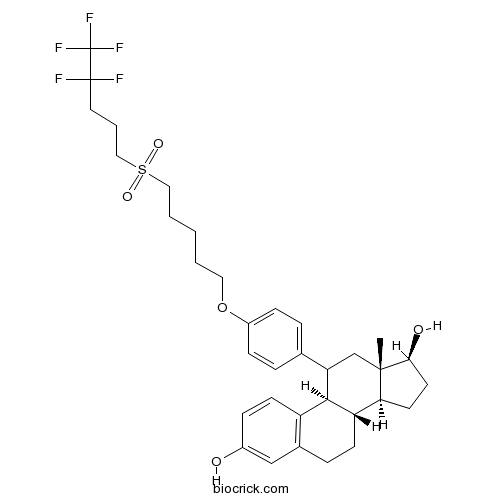
| Cas No. | 151555-47-4 | SDF | Download SDF |
| PubChem ID | 119604 | Appearance | Powder |
| Formula | C34H43F5O5S | M.Wt | 658.76 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| Chemical Name | (8S,9R,13S,14S,17S)-13-methyl-11-[4-[5-(4,4,5,5,5-pentafluoropentylsulfonyl)pentoxy]phenyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol | ||
| SMILES | CC12CC(C3C(C1CCC2O)CCC4=C3C=CC(=C4)O)C5=CC=C(C=C5)OCCCCCS(=O)(=O)CCCC(C(F)(F)F)(F)F | ||
| Standard InChIKey | SDCUWFRXMLQNCS-JGNCIJAKSA-N | ||
| Standard InChI | InChI=1S/C34H43F5O5S/c1-32-21-28(31-26-13-9-24(40)20-23(26)8-12-27(31)29(32)14-15-30(32)41)22-6-10-25(11-7-22)44-17-3-2-4-18-45(42,43)19-5-16-33(35,36)34(37,38)39/h6-7,9-11,13,20,27-31,40-41H,2-5,8,12,14-19,21H2,1H3/t27-,28?,29-,30-,31+,32-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pure antiestrogen that downregulates estrogen receptor expression (IC50 = 0.04 nM). Displays potent antiproliferative activity in vitro (IC50 = 0.035 - 0.09 nM in MCF-7 cells) and causes long term regression of tamoxifen-resistant MCF-7 xenografts in vivo. |

RU 58668 Dilution Calculator

RU 58668 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.518 mL | 7.59 mL | 15.18 mL | 30.3601 mL | 37.9501 mL |
| 5 mM | 0.3036 mL | 1.518 mL | 3.036 mL | 6.072 mL | 7.59 mL |
| 10 mM | 0.1518 mL | 0.759 mL | 1.518 mL | 3.036 mL | 3.795 mL |
| 50 mM | 0.0304 mL | 0.1518 mL | 0.3036 mL | 0.6072 mL | 0.759 mL |
| 100 mM | 0.0152 mL | 0.0759 mL | 0.1518 mL | 0.3036 mL | 0.3795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Levomefolate calcium
Catalog No.:BCC1702
CAS No.:151533-22-1
- N-[[1-[(2-Nitrophenyl)sulfonyl]-1H-indole-3-yl]methyl]-N-[1-[1-[(2-nitrophenyl)sulfonyl]-1H-indole-3-yl]-2-oxo-2-(tert-butylamino)ethyl]-1-(2-diazo-3-oxobutyryl)-2-oxo-3-methylpiperidine-3beta-carboxamide
Catalog No.:BCC8335
CAS No.:151513-70-1
- Ampelopsin F
Catalog No.:BCN3305
CAS No.:151487-08-0
- Swietemahalactone
Catalog No.:BCN6886
CAS No.:1514669-21-6
- Estradiol hexahydrobenzoate
Catalog No.:BCC8962
CAS No.:15140-27-9
- Pseudolarolide B
Catalog No.:BCN8093
CAS No.:151368-43-3
- Ro 32-0432 hydrochloride
Catalog No.:BCC7122
CAS No.:151342-35-7
- 3-O-p-Coumaroyloleanolic acid
Catalog No.:BCN3952
CAS No.:151334-06-4
- Zaleplon
Catalog No.:BCC5197
CAS No.:151319-34-5
- Inokosterone
Catalog No.:BCN3431
CAS No.:15130-85-5
- Genkwanol C
Catalog No.:BCN8012
CAS No.:151283-11-3
- CP 135807
Catalog No.:BCC7774
CAS No.:151272-90-1
- ent-3-Oxokauran-17-oic acid
Catalog No.:BCN1674
CAS No.:151561-88-5
- JJKK 048
Catalog No.:BCC5610
CAS No.:1515855-97-6
- XEN445
Catalog No.:BCC5382
CAS No.:1515856-92-4
- 6-Prenylquercetin-3-methylether
Catalog No.:BCN7992
CAS No.:151649-34-2
- H-D-Ser-OBzl.HCl
Catalog No.:BCC3097
CAS No.:151651-44-4
- SEP-0372814
Catalog No.:BCC6429
CAS No.:1516895-53-6
- 5-Deoxystrigol
Catalog No.:BCN7693
CAS No.:151716-18-6
- 2,2-Dimethyl-8-prenylchromene 6-carboxylic acid
Catalog No.:BCN1675
CAS No.:151731-50-9
- 4,4'-Dihydroxy-2,6-dimethoxydihydrochalcone
Catalog No.:BCN3583
CAS No.:151752-08-8
- Montelukast Sodium
Catalog No.:BCC4680
CAS No.:151767-02-1
- AC 187
Catalog No.:BCC6018
CAS No.:151804-77-2
- cAMPS-Rp, triethylammonium salt
Catalog No.:BCC8082
CAS No.:151837-09-1
Pure antiestrogen RU 58668-loaded nanospheres: morphology, cell activity and toxicity studies.[Pubmed:14757510]
Eur J Pharm Sci. 2004 Feb;21(2-3):361-70.
Nanospheres (NS) formulated using biodegradable and biocompatible polymers, poly(D,L-lactide-co-glycolide) (PLGA), poly(D,L-lactide) (PLA) and poly(epsilon-caprolactone) (PCL), loaded with the pure anti-estrogen RU 58668 (RU), a promising estrogen-dependent anticancer agent, have been prepared. They all possess a small size compatible with an intratumoral extravasation behavior and their pegylation reduce significantly their zeta potential. Characterization by freeze fracture electron microscopy have shown that NS are spheric particles with a size ranging between 30 and 50nm and a tendency to agglomerate which is reduced by polyethylene glycol (PEG) grafting. PEG-grafted NS are all non-toxic as revealed by cell viability assay. A specific cellular model has been used to evaluate not only the release extent of the drug but also its biological activity. All formulations tested showed that they release slowly RU as measured by the delayed ability of RU to inhibit estrogen-induced transcription in human breast cancer cells and that they possess only a small amount of surface adsorbed RU.
Polyester-poly(ethylene glycol) nanoparticles loaded with the pure antiestrogen RU 58668: physicochemical and opsonization properties.[Pubmed:12880293]
Pharm Res. 2003 Jul;20(7):1063-70.
PURPOSE: The pure antiestrogen RU58668 (RU) was encapsulated within nanospheres (NS) and nanocapsules (NC) prepared from different polyester copolymers with poly(ethylene glycol) (PEG) chains. The influence of their physicochemical properties on drug release in vitro and their susceptibility to opsonization were evaluated. METHODS: RU-loaded PEG-bearing nanoparticles (NP) prepared by interfacial deposition of preformed polymer were characterized (size, zeta potential, percentage encapsulation and loading). In vitro release kinetics were studied in the presence of 10% fetal calf serum (FCS). Their opsonization in mouse serum was evaluated by silver staining of SDS-PAGE and Western blotting of desorbed proteins. RESULTS: The NS were smaller than NC and had a zeta potential close to zero and a higher percentage of loading. RU release from NS in vitro was reduced as compared with the dissolution profile of free RU in a serum-containing medium. Decreased opsonin adsorption at the surface of pegylated NS was observed. CONCLUSION: Small nanoparticulate systems containing a high load of pure antiestrogen, showing reduced drug release, have been developed. Among the six nanosphere preparations containing RU, two show a size below 200 nm, and two others undergo reduced protein adsorption in the presence of serum, compatible with increased persistence in the blood.
In vitro and in vivo biologic evaluation of long-circulating biodegradable drug carriers loaded with the pure antiestrogen RU 58668.[Pubmed:12845687]
Int J Cancer. 2003 Sep 1;106(3):446-54.
We have developed a parenteral delivery system for the administration of the highly promising pure antiestrogen RU 58668 (RU). Two types of nanoparticles (NP) made of biodegradable copolymers and coated with polyethylene-glycol (PEG) chains were prepared: nanospheres (NS) (diameter, approximately 110 nm) and nanocapsules (NC) with an oily core (diameter, approximately 250 nm). The amount of RU incorporated into NS and NC was approximately 33 vs. approximately 5 microg RU/mg of polymer, respectively. Coating with PEG chains prolonged the antiestrogenic potency of RU, as shown by a prolonged antiuterotrophic activity of encapsulated RU into PEG-poly(D,L lactic acid) (PLA) NS, as compared to that of conventional nonpegylated NS. In mice bearing MCF-7 estrogen-dependent tumors, free RU injected at 4.3 mg/kg/week by i.v. route slightly decreased the estradiol-promoted (0.5 mg/kg/week) tumor growth while RU-loaded PEG-PLA NS injected at the same dose strongly reduced it. Analysis of cell cycle parameters in tumors treated with RU indicated that RU-loaded PEG-PLA NS injected at 4.3 mg/kg/week in MCF-7 tumors decreased cyclin D(1) and cyclin E simultaneously, and increased p27. The antitumoral activity of RU encapsulated within pegylated NC was stronger than that of RU entrapped with pegylated NS loaded at an equivalent dose. Indeed, the former decreased the tumor size in nude mice transplanted with the estrogen receptor-positive but estrogen-independent MCF-7/Ras breast cancer cells at a concentration 2.5 times lower than that of the latter (0.4 mg/kg/week compared to 1 mg/kg/week). Empty PEG-PLA NS and NC were devoid of antiuterotrophic and antitumoral activities. Altogether, these results suggest that the incorporation of the pure antiestrogen RU into long-circulating NP could represent a novel antiestrogen drug delivery system for the parenteral route.
Improved antitumoral properties of pure antiestrogen RU 58668-loaded liposomes in multiple myeloma.[Pubmed:16753295]
J Steroid Biochem Mol Biol. 2006 Jul;100(1-3):67-78.
In most of multiple myeloma (MM) cells, the "pure" antiestrogen (AE) RU 58668 (RU) induced either a G1-arrest (LP-1, OPM-2, NCI-H929, U266 cells) or apoptosis (RPMI 8226 cells). In RPMI 8226 cells, RU activates a caspase-dependent cell death pathway leading to the release of cytochrome c, the decrease of the essential MM survival factor Mcl-1, the cleavage of Bid and the activation of caspases-3 and -8. Incorporation of RU in pegylated cholesterol-containing liposomes allowed a controlled RU release, improving its anti-proliferative and apoptotic effects in cells. In RPMI 8226 xenografts, i.v. injected RU-liposomes but not free RU, exhibited antitumor activity. In vivo, RU-liposomes triggered the mitochondrial death pathway, concomitantly with a down-regulation of Mcl-1 and Bid cleavage. The decrease of CD34 immunoreactivity indicated a reduction of angiogenesis. The decrease of VEGF secretion in vitro supported a direct effect of RU on angiogenesis. These pro-apoptotic and antiangiogenic effects were explained by a prolonged exposure to the drug and to the endocytosis capacity of liposomes which might increase RU uptake and bypass a membrane export of free RU. Thus, these combined enhanced activities of RU-liposomes support that such a delivery of an AE may constitute a strategy of benefit for MM treatment.
Partial antagonism between steroidal and nonsteroidal antiestrogens in human breast cancer cell lines.[Pubmed:9443403]
Cancer Res. 1998 Jan 15;58(2):263-7.
Nonsteroidal antiestrogens, such as tamoxifen, are well established in the treatment of breast cancer. The development of new steroidal compounds without partial agonist activity allows deeper insights into the mechanism of antiestrogen action, but thus far, the combined use of steroidal and nonsteroidal antiestrogens has not been studied extensively. We compared the nonsteroidal 4-trans-hydroxytamoxifen (OHT) with the two steroidal antiestrogens, ICI 182780 and RU 58668, in the estrogen receptor-positive human breast cancer cell lines MCF-7 and T47D. The effect of each compound alone or of OHT in combination with one of the steroidal antiestrogens was studied in regard to cell proliferation, expression of estrogen receptors (ERs) and progesterone receptors, and secretion of transforming growth factor beta2 (TGF-beta2). All antiestrogens examined led to enhanced secretion of TGF-beta2, which is correlated with their individual growth-inhibitory potential. OHT partially counteracts the larger growth inhibition of human breast cancer cells exerted by the steroidal antiestrogens ICI 182780 and RU 58668. Also, OHT antagonizes the higher induction of TGF-beta2 seen after treatment of MCF-7 cells with steroidal antiestrogens. The loss of ER and down-regulation of progesterone receptor under treatment with the steroidal antiestrogens is prevented by OHT, whereas the steroidal antiestrogens prevent the ability of hydroxytamoxifen to increase the ER content. These results indicate that TGF-beta2 is a marker of action for both types of compounds, but steroidal and nonsteroidal antiestrogens partially antagonize each other in blocking ER-mediated cellular events. It would appear that no additive or synergistic effect of the two types of antiestrogens can be expected in the treatment of breast cancer.


