RO-9187HCV virus replication inhibitor CAS# 876708-03-1 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- WHI-P180 hydrochloride
Catalog No.:BCC4243
CAS No.:153437-55-9
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
Number of papers citing our products

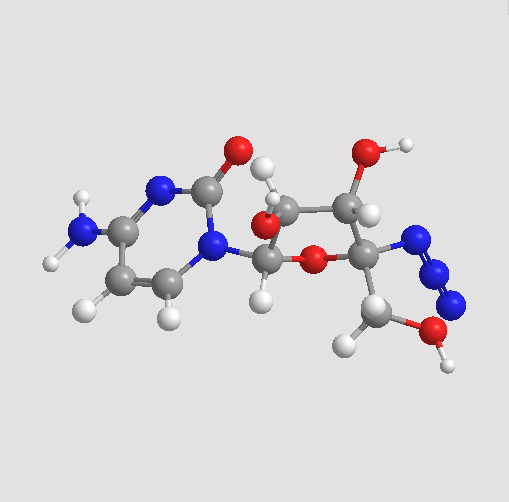
Chemical structure
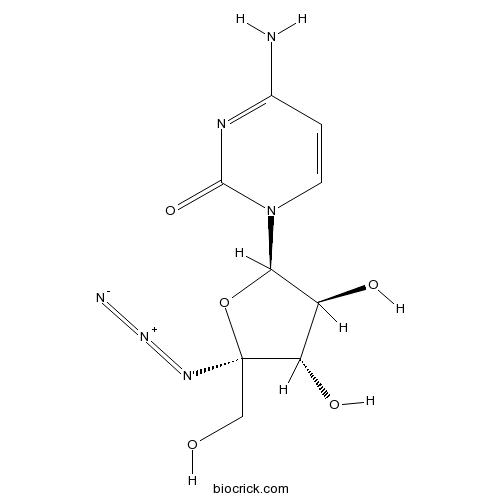
3D structure
| Cas No. | 876708-03-1 | SDF | Download SDF |
| PubChem ID | 11514721 | Appearance | Powder |
| Formula | C9H12N6O5 | M.Wt | 284.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 7.14 mg/mL (25.12 mM; Need ultrasonic) | ||
| Chemical Name | 4-amino-1-[(2R,3S,4S,5R)-5-azido-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one | ||
| SMILES | C1=CN(C(=O)N=C1N)C2C(C(C(O2)(CO)N=[N+]=[N-])O)O | ||
| Standard InChIKey | ODLGMSQBFONGNG-XZMZPDFPSA-N | ||
| Standard InChI | InChI=1S/C9H12N6O5/c10-4-1-2-15(8(19)12-4)7-5(17)6(18)9(3-16,20-7)13-14-11/h1-2,5-7,16-18H,3H2,(H2,10,12,19)/t5-,6-,7+,9+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RO-9187 is a potent inhibitor of HCV virus replication with an IC50 of 171 nM.In Vitro:RO-9187 is excellent substrates for deoxycytidine kinase and is phosphorylated with efficiencies up to 3-fold higher than deoxycytidine. RO-9187 inhibits RNA synthesis by HCV polymerases from either HCV genotypes 1a and 1b or containing S96T or S282T point mutations with similar potencies, suggesting no cross-resistance with either R1479 (4′-azidocytidine) or 2′-C-methyl nucleosides. The formation of RO-9187-TP increased in a time- and dose-dependent manner. The maximal triphosphate concentration at 24 h is 87 pmol/106 cells with half-maximal triphosphate formation achieved at 12 μM RO-9187[1].In Vivo:Plasma exposures of RO-9187 in rats increase in a dose-dependent manner between 10 and 2000 mg/kg after oral dosing. Plasma concentrations of 1.4 and 26 μM (390 and 7454 ng/mL) are achieved in rats and dogs at the 10 mg/kg dose level, respectively. Plasma concentrations up to 57 μM are achieved in rats dosed with 2000 mg/kg/day[1]. References: | |||||

RO-9187 Dilution Calculator

RO-9187 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5183 mL | 17.5914 mL | 35.1828 mL | 70.3655 mL | 87.9569 mL |
| 5 mM | 0.7037 mL | 3.5183 mL | 7.0366 mL | 14.0731 mL | 17.5914 mL |
| 10 mM | 0.3518 mL | 1.7591 mL | 3.5183 mL | 7.0366 mL | 8.7957 mL |
| 50 mM | 0.0704 mL | 0.3518 mL | 0.7037 mL | 1.4073 mL | 1.7591 mL |
| 100 mM | 0.0352 mL | 0.1759 mL | 0.3518 mL | 0.7037 mL | 0.8796 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RO-9187 is a potent inhibitor of HCV virus replication in the replicon system (IC(50) = 171 +/- 12 nM; CC(50) >1 mM). Pharmacokinetic studies with RO-9187 in rats and dogs showed that plasma concentrations exceeding HCV replicon IC(50) values 8-150-fold could be achieved by low dose (10 mg/kg) oral administration.
- RF 9
Catalog No.:BCC7744
CAS No.:876310-60-0
- Montixanthone
Catalog No.:BCN8069
CAS No.:876305-36-1
- Ptaquiloside
Catalog No.:BCN8159
CAS No.:87625-62-5
- Smyrindioloside
Catalog No.:BCN4423
CAS No.:87592-77-6
- (+)-Lyoniresinol 9'-O-glucoside
Catalog No.:BCN4832
CAS No.:87585-32-8
- LXR-623
Catalog No.:BCC4273
CAS No.:875787-07-8
- cis-3,4-Dihydroxy-beta-ionone
Catalog No.:BCN6694
CAS No.:875666-39-0
- PPY A
Catalog No.:BCC3895
CAS No.:875634-01-8
- Kelampayoside A
Catalog No.:BCN4422
CAS No.:87562-76-3
- Randaiol
Catalog No.:BCN4002
CAS No.:87562-14-9
- ent-14,15-Dinor-13-oxolabda-8(17),11-dien-18-oic acid
Catalog No.:BCN1319
CAS No.:875585-30-1
- H-D-Tyr-OtBu
Catalog No.:BCC3136
CAS No.:87553-74-0
- Trandolapril
Catalog No.:BCC5275
CAS No.:87679-37-6
- 6-Hydroxyrubiadin
Catalog No.:BCN4425
CAS No.:87686-86-0
- Pentoxyresorufin
Catalog No.:BCC6297
CAS No.:87687-03-4
- Isomagnolol
Catalog No.:BCN8325
CAS No.:87688-90-2
- 3-(1-Piperazinyl)-1,2-benzisothiazole
Catalog No.:BCC8585
CAS No.:87691-87-0
- Alismoxide
Catalog No.:BCN1265
CAS No.:87701-68-6
- GPBAR-A
Catalog No.:BCC6201
CAS No.:877052-79-4
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
- H-Tyrosinol
Catalog No.:BCC2697
CAS No.:87745-27-5
- Bryostatin 2
Catalog No.:BCC5619
CAS No.:87745-28-6
- Tandospirone
Catalog No.:BCC4208
CAS No.:87760-53-0
- ML 221
Catalog No.:BCC6278
CAS No.:877636-42-5
Escape of Tick-Borne Flavivirus from 2'-C-Methylated Nucleoside Antivirals Is Mediated by a Single Conservative Mutation in NS5 That Has a Dramatic Effect on Viral Fitness.[Pubmed:28814513]
J Virol. 2017 Oct 13;91(21). pii: JVI.01028-17.
Tick-borne encephalitis virus (TBEV) causes a severe and potentially fatal neuroinfection in humans. Despite its high medical relevance, no specific antiviral therapy is currently available. Here we demonstrate that treatment with a nucleoside analog, 7-deaza-2'-C-methyladenosine (7-deaza-2'-CMA), substantially improved disease outcomes, increased survival, and reduced signs of neuroinfection and viral titers in the brains of mice infected with a lethal dose of TBEV. To investigate the mechanism of action of 7-deaza-2'-CMA, two drug-resistant TBEV clones were generated and characterized. The two clones shared a signature amino acid substitution, S603T, in the viral NS5 RNA-dependent RNA polymerase (RdRp) domain. This mutation conferred resistance to various 2'-C-methylated nucleoside derivatives, but no cross-resistance was seen with other nucleoside analogs, such as 4'-C-azidocytidine and 2'-deoxy-2'-beta-hydroxy-4'-azidocytidine (RO-9187). All-atom molecular dynamics simulations revealed that the S603T RdRp mutant repels a water molecule that coordinates the position of a metal ion cofactor as 2'-C-methylated nucleoside analogs approach the active site. To investigate its phenotype, the S603T mutation was introduced into a recombinant TBEV strain (Oshima-IC) generated from an infectious cDNA clone and into a TBEV replicon that expresses a reporter luciferase gene (Oshima-REP-luc2A). The mutants were replication impaired, showing reduced growth and a small plaque size in mammalian cell culture and reduced levels of neuroinvasiveness and neurovirulence in rodent models. These results indicate that TBEV resistance to 2'-C-methylated nucleoside inhibitors is conferred by a single conservative mutation that causes a subtle atomic effect within the active site of the viral NS5 RdRp and is associated with strong attenuation of the virus.IMPORTANCE This study found that the nucleoside analog 7-deaza-2'-C-methyladenosine (7-deaza-2'-CMA) has high antiviral activity against tick-borne encephalitis virus (TBEV), a pathogen that causes severe human neuroinfections in large areas of Europe and Asia and for which there is currently no specific therapy. Treating mice infected with a lethal dose of TBEV with 7-deaza-2'-CMA resulted in significantly higher survival rates and reduced the severity of neurological signs of the disease. Thus, this compound shows promise for further development as an anti-TBEV drug. It is important to generate drug-resistant mutants to understand how the drug works and to develop guidelines for patient treatment. We generated TBEV mutants that were resistant not only to 7-deaza-2'-CMA but also to a broad range of other 2'-C-methylated antiviral medications. Our findings suggest that combination therapy may be used to improve treatment and reduce the emergence of drug-resistant viruses during nucleoside analog therapy for TBEV infection.
2'-deoxy-4'-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2'-alpha-hydroxyl groups.[Pubmed:18003608]
J Biol Chem. 2008 Jan 25;283(4):2167-75.
RNA polymerases effectively discriminate against deoxyribonucleotides and specifically recognize ribonucleotide substrates most likely through direct hydrogen bonding interaction with the 2'-alpha-hydroxy moieties of ribonucleosides. Therefore, ribonucleoside analogs as inhibitors of viral RNA polymerases have mostly been designed to retain hydrogen bonding potential at this site for optimal inhibitory potency. Here, two novel nucleoside triphosphate analogs are described, which are efficiently incorporated into nascent RNA by the RNA-dependent RNA polymerase NS5B of hepatitis C virus (HCV), causing chain termination, despite the lack of alpha-hydroxy moieties. 2'-deoxy-2'-beta-fluoro-4'-azidocytidine (RO-0622) and 2'-deoxy-2'-beta-hydroxy-4'-azidocytidine (RO-9187) were excellent substrates for deoxycytidine kinase and were phosphorylated with efficiencies up to 3-fold higher than deoxycytidine. As compared with previous reports on ribonucleosides, higher levels of triphosphate were formed from RO-9187 in primary human hepatocytes, and both compounds were potent inhibitors of HCV virus replication in the replicon system (IC(50) = 171 +/- 12 nM and 24 +/- 3 nM for RO-9187 and RO-0622, respectively; CC(50) >1 mM for both). Both compounds inhibited RNA synthesis by HCV polymerases from either HCV genotypes 1a and 1b or containing S96T or S282T point mutations with similar potencies, suggesting no cross-resistance with either R1479 (4'-azidocytidine) or 2'-C-methyl nucleosides. Pharmacokinetic studies with RO-9187 in rats and dogs showed that plasma concentrations exceeding HCV replicon IC(50) values 8-150-fold could be achieved by low dose (10 mg/kg) oral administration. Therefore, 2'-alpha-deoxy-4'-azido nucleosides are a new class of antiviral nucleosides with promising preclinical properties as potential medicines for the treatment of HCV infection.


