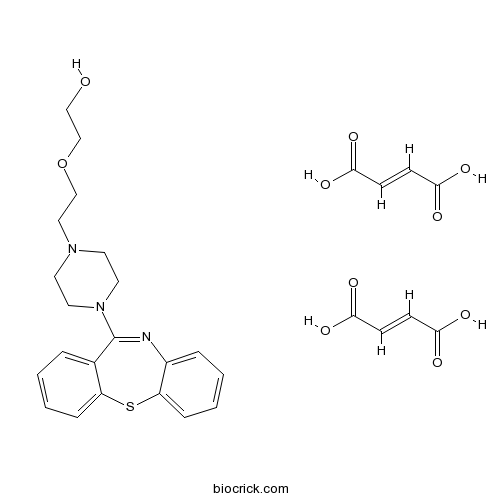
Quetiapine fumarate5-HT2/D2 antagonist; atypical antipsychotic CAS# 111974-72-2 |
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- TGX-221
Catalog No.:BCC1244
CAS No.:663619-89-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 111974-72-2 | SDF | Download SDF |
| PubChem ID | 6436253 | Appearance | Powder |
| Formula | C29H33N3O10S | M.Wt | 615.7 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | ICI 204,636 | ||
| Solubility | DMSO : 50 mg/mL (113.24 mM; Need ultrasonic) H2O : 1.25 mg/mL (2.83 mM; Need ultrasonic) | ||
| Chemical Name | 2-[2-(4-benzo[b][1,4]benzothiazepin-6-ylpiperazin-1-yl)ethoxy]ethanol;(E)-but-2-enedioic acid | ||
| SMILES | C1CN(CCN1CCOCCO)C2=NC3=CC=CC=C3SC4=CC=CC=C42.C(=CC(=O)O)C(=O)O.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | JLWSQVHJLLYDPX-LVEZLNDCSA-N | ||
| Standard InChI | InChI=1S/C21H25N3O2S.2C4H4O4/c25-14-16-26-15-13-23-9-11-24(12-10-23)21-17-5-1-3-7-19(17)27-20-8-4-2-6-18(20)22-21;2*5-3(6)1-2-4(7)8/h1-8,25H,9-16H2;2*1-2H,(H,5,6)(H,7,8)/b;2*2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Quetiapine fumarate monotherapy (150 mg/day and 300 mg/day) is effective, with safety and tolerability in the treatment of patients with major depressive disorder. 2. Quetiapine fumarate can treat patients with schizophrenia. |
| Targets | Androgen Receptor | Estrogen receptor | Progestogen receptor |

Quetiapine fumarate Dilution Calculator

Quetiapine fumarate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6242 mL | 8.1208 mL | 16.2417 mL | 32.4834 mL | 40.6042 mL |
| 5 mM | 0.3248 mL | 1.6242 mL | 3.2483 mL | 6.4967 mL | 8.1208 mL |
| 10 mM | 0.1624 mL | 0.8121 mL | 1.6242 mL | 3.2483 mL | 4.0604 mL |
| 50 mM | 0.0325 mL | 0.1624 mL | 0.3248 mL | 0.6497 mL | 0.8121 mL |
| 100 mM | 0.0162 mL | 0.0812 mL | 0.1624 mL | 0.3248 mL | 0.406 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Quetiapine fumarate is an atypical antipsychotic used in the treatment of schizophrenia, bipolar I mania, bipolar II depression, bipolar I depression.
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- Endoxifen
Catalog No.:BCC7761
CAS No.:112093-28-4
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
Extended release quetiapine fumarate monotherapy in major depressive disorder: a placebo- and duloxetine-controlled study.[Pubmed:19358790]
J Clin Psychiatry. 2009 Apr;70(4):526-39. Epub 2009 Apr 7.
OBJECTIVE: To evaluate the efficacy and tolerability of once-daily extended release Quetiapine fumarate (quetiapine XR) as monotherapy treatment for major depressive disorder (MDD). METHOD: This 8-week (6-week active-treatment, randomized phase; 2-week posttreatment drug-discontinuation/tapering phase), multicenter, double-blind, randomized, parallel-group, placebo- and active-controlled, phase 3 study was conducted between April 2006 and May 2007. In total, 612 patients with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)-defined MDD were randomly assigned to quetiapine XR 150 mg/day or 300 mg/day, duloxetine 60 mg/day (active control), or placebo. The primary endpoint was the change from baseline to week 6 in Montgomery-Asberg Depression Rating Scale (MADRS) total score. RESULTS: At week 6, both doses of quetiapine XR (p < .001) and duloxetine (p < .01) significantly reduced mean MADRS total score versus placebo. A significant reduction was seen at week 1 with quetiapine XR 150 mg/day and 300 mg/day versus placebo (p < .01), but not with duloxetine. Response rates (>or= 50% reduction in MADRS total score) at week 6 were significantly higher for both doses of quetiapine XR (p < .01) and duloxetine (p < .05) versus placebo. Remission rates (MADRS score OBJECTIVES: This study aimed to demonstrate efficacy of once-daily extended release Quetiapine fumarate (quetiapine XR) versus placebo in patients with acute schizophrenia. METHODS: In this 6-week, randomized, double-blind study (5077IL/0041) patients were randomized to receive quetiapine XR (300, 600, or 800 mg/day), Quetiapine fumarate immediate release (quetiapine IR) [300 or 600 mg/day], or placebo. Primary endpoint was change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score at Day 42. Secondary variables included PANSS response rate at Day 42 (>/=30% decrease in PANSS total score from baseline) and Clinical Global Impressions Severity (CGI-S) and Improvement (CGI-I) ratings. Safety assessments included adverse event (AE) reporting and laboratory measures. RESULTS: Of 532 patients randomized, 222 (41.7%) completed the study. Improvements in PANSS total scores from baseline to Day 42 across treatment groups were: quetiapine XR 300 mg/day -5.01, 600 mg/day -13.01 and 800 mg/day -11.17, quetiapine IR 300 mg/day -9.42 and 600 mg/day -6.97, and placebo -5.19; the difference in change was statistically significant only for quetiapine XR 600 mg/day (p = 0.033). There were no statistically significant differences between active treatment groups and placebo for PANSS response rates. Several post hoc analyses were conducted to explain the study efficacy outcome but these were inconclusive. Quetiapine XR was generally well tolerated with the majority of AEs being mild or moderate in intensity and no unexpected AEs. CONCLUSIONS: Superior efficacy of quetiapine XR versus placebo in patients with schizophrenia was demonstrated for quetiapine XR 600 mg/day. The safety and tolerability profile of quetiapine XR was similar to that of quetiapine IR. BACKGROUND AND OBJECTIVE: The extended-release formulation of quetiapine (quetiapine XR), which was developed to provide more convenient once-daily administration, has been widely studied to characterize its pharmacokinetics in Caucasian populations but has rarely been studied in an Asia population. This study was conducted to evaluate the pharmacokinetics and tolerability of quetiapine XR administered as a single dose (300 mg) and multiple doses (300, 600, and 800 mg) in Han Chinese patients with schizophrenia. METHODS: This was a single-center, open-label, single-dose and multiple-dose randomized study. Among the 55 randomized subjects, a total of 40 female or male patients in 300 mg (n = 13), 600 mg (n = 13), or 800 mg (n = 14) groups completed the study of Quetiapine fumarate XR. The treatment phase consisted of 5 consecutive days and was preceded by a 1- to 2-day titration period for the 600 and 800 mg groups. Pharmacokinetic parameters for both quetiapine and N-desalkyl quetiapine (norquetiapine) were determined. The tolerability evaluation included adverse events (AEs) noted by monitoring, physical examinations, vital signs, and clinical laboratory tests. RESULTS: N-desalkyl quetiapine was formed from quetiapine with an approximate metabolite to parent ratio of 0.5 across the three dose groups. The geometric mean elimination half-life (t (1/2)) of both quetiapine and N-desalkyl quetiapine was consistent for the three dosing groups (approximately 7 h for quetiapine and approximately 18 h for N-desalkyl quetiapine). The geometric mean maximum plasma concentrations (C max) at steady state (C max,ss) of quetiapine for the three groups were 467, 740, and 1,126 ng/mL, respectively, and for N-desalkyl quetiapine were 138, 262, and 426 ng/mL, respectively. The values for the geometric mean area under the plasma concentration-time curve over a dosing interval at the steady-state (AUCss) of quetiapine were 5,094, 7,685, and 13,237 ng.h/mL, respectively, and for N-desalkyl quetiapine were 2,284, 4,341, and 7,216 ng.h/mL, respectively. The apparent oral clearance (CL/F) of quetiapine at steady state appeared to be comparable across the three dose groups. The pharmacokinetics of quetiapine XR were dose-proportional across the dosage range employed. The most common AE was somnolence, but all of the reported AEs were mild. There were no serious AEs or other significant AEs. CONCLUSION: Quetiapine fumarate XR has a dose-proportional pharmacokinetic profile at doses ranging from 300 to 800 mg once daily, and a slower time to reach C max and steady state after 3 days of sequential dosing. Therefore, it offers a simple and rapid dose-escalation option and more convenient once-daily administration. The three dosages of Quetiapine fumarate XR were generally well-tolerated in this pharmacokinetic study of Han Chinese patients with schizophrenia. Multiple sclerosis (MS) is a central nervous system disorder that is associated with progressive oligodendrocyte and neuronal loss, axonal degeneration, and demyelination. Several medications that mitigate immune abnormalities reduce both the frequency of relapses and inflammation on magnetic resonance imaging, leading to improved outcomes for people with the relapsing-remitting form of MS. However, there are no treatments for the progressive forms of MS where neurons and axons continue to degenerate; here, neuroprotective therapies, or medications that rebuild myelin to confer axonal well-being, may be useful. Quetiapine fumarate is an atypical antipsychotic with reported remyelinating and neuroprotective properties in inflammatory and noninflammatory models of demyelination, including experimental autoimmune encephalomyelitis, and both cuprizone- and global cerebral ischemia-induced demyelination. Preclinical studies suggest that quetiapine may exert these effects by stimulating proliferation and maturation of oligodendrocytes, releasing neurotrophic factors, increasing antioxidant defences, scavenging for free radicals, and inhibiting activated microglia, astrocytes, and T lymphocytes. Additionally, quetiapine may be beneficial for psychiatric and nonpsychiatric symptoms of MS including depression, anxiety, insomnia, and possibly even pain. These data indicate that clinical trials are justified to determine the safety, tolerability, and efficacy of Quetiapine fumarate in MS. The pharmacologic treatment of schizophrenia still suffers from two major problems: (1) most antipsychotic drugs still induce severe neurologic (extrapyramidal) side effects; (2) few antipsychotic drugs are effective in treating the negative symptoms of schizophrenia. In the present study, we have evaluated the effects of ICI 204,636 in the rat paw test and the amphetamine-induced social isolation in monkeys and compared them with the effects of clozapine. The paw test has been shown to be a valid model for differentiating classic and atypical neuroleptic drugs. The monkey social isolation model seems to represent one of the few animal models with validity for the negative symptoms of schizophrenia. The results show that both ICI 204,636 and clozapine had the profile of an atypical antipsychotic in the paw test, suggesting a reduced propensity to induce extrapyramidal side effects in humans. Likewise, ICI 204,636 and clozapine were found to prevent the amphetamine-induced social isolation in monkeys, suggesting a good therapeutic effect mitigating the negative symptoms in schizophrenia. Overall, the data suggest that ICI 204,636 may represent a new and interesting antipsychotic drug, closely resembling clozapine. Seroquel and the atypical antipsychotic clozapine were compared using a number of biochemical measures in rats which are indicative of potential antipsychotic activity and possible extrapyramidal side effect liability. Both in vitro and in vivo, these compounds are low potency D-2 dopamine (DA) receptor antagonists and are relatively more potent 5-HT2 antagonists than typical antipsychotic drugs. Seroquel also exhibited low affinity for D-1 DA receptors in vitro, but D-1 receptor occupancy was not detectable in vivo. Unlike clozapine, Seroquel lacks appreciable activity at either D-1 DA or muscarinic receptors. Following IP administration, both compounds produce similar elevations in DA metabolite concentrations. Following 1 month of daily administration, at doses which produce large increases in striatal DA metabolite concentrations, both Seroquel and clozapine fail, unlike typical antipsychotics, to increase the number of striatal D-2 receptors, but do decrease the number of 5-HT2 receptors in frontal cortex. ICI 204,636 produces a short-lasting increase in plasma prolactin levels, but these increases are much greater than those that are produced by clozapine. One day after 3 weeks of daily administration, tolerance, to the ability of Seroquel to elevate DA metabolite and plasma PRL concentrations is not observed. These biochemical observations are discussed with regard to the atypical profile of Seroquel in behavioral and electrophysiological studies.The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study.[Pubmed:18779774]
Psychopharmacol Bull. 2008;41(3):11-35.
Pharmacokinetics and tolerability of extended-release quetiapine fumarate in Han Chinese patients with schizophrenia.[Pubmed:24385309]
Clin Pharmacokinet. 2014 May;53(5):455-65.
Quetiapine fumarate for the treatment of multiple sclerosis: focus on myelin repair.[Pubmed:23870612]
CNS Neurosci Ther. 2013 Oct;19(10):737-44.
Activity of "seroquel" (ICI 204,636) in animal models for atypical properties of antipsychotics: a comparison with clozapine.[Pubmed:8887995]
Neuropsychopharmacology. 1996 Oct;15(4):406-16.
Seroquel: biochemical profile of a potential atypical antipsychotic.[Pubmed:7871032]
Psychopharmacology (Berl). 1993;112(2-3):285-92.


