PyridostatinDrug used for promoting growth arrest CAS# 1085412-37-8 |
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- 360A iodide
Catalog No.:BCC1308
CAS No.:737763-37-0
- 360A
Catalog No.:BCC1307
CAS No.:794458-56-3
- AT13387
Catalog No.:BCC2122
CAS No.:912999-49-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1085412-37-8 | SDF | Download SDF |
| PubChem ID | 25227847 | Appearance | Powder |
| Formula | C31H32N8O5 | M.Wt | 596.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RR82 | ||
| Solubility | Soluble to 100 mM in sterile water | ||
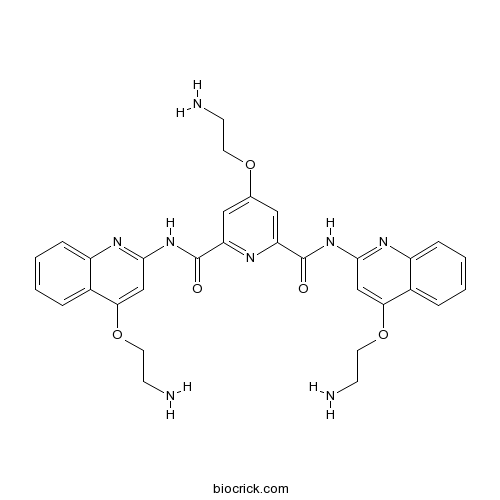
| Chemical Name | 4-(2-aminoethoxy)-2-N,6-N-bis[4-(2-aminoethoxy)quinolin-2-yl]pyridine-2,6-dicarboxamide | ||
| SMILES | C1=CC=C2C(=C1)C(=CC(=N2)NC(=O)C3=CC(=CC(=N3)C(=O)NC4=NC5=CC=CC=C5C(=C4)OCCN)OCCN)OCCN | ||
| Standard InChIKey | VGHSATQVJCTKEF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H32N8O5/c32-9-12-42-19-15-24(30(40)38-28-17-26(43-13-10-33)20-5-1-3-7-22(20)36-28)35-25(16-19)31(41)39-29-18-27(44-14-11-34)21-6-2-4-8-23(21)37-29/h1-8,15-18H,9-14,32-34H2,(H,36,38,40)(H,37,39,41) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pyridostatin is a G-quadruplexe stabilizer, with a Kd of 490 nM.In Vitro:Pyridostatin is a G-quadruplexe stabilizer, with a Kd of 490 nM[1]. Pyridostatin (PDS) shows neurotoxic activity against primary cortical neurons at 0.01-5 μM, causes DNA double-strand breaks (DSBs) at 1 μM, downregulates BRCA1 in neurons at 1, 2 or 5 μM[2]. Pyridostatin interacts with G-quadruplex motifs in SRC and alters mRNA levels of damaged genes[3]. References: | |||||

Pyridostatin Dilution Calculator

Pyridostatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6761 mL | 8.3803 mL | 16.7605 mL | 33.5211 mL | 41.9013 mL |
| 5 mM | 0.3352 mL | 1.6761 mL | 3.3521 mL | 6.7042 mL | 8.3803 mL |
| 10 mM | 0.1676 mL | 0.838 mL | 1.6761 mL | 3.3521 mL | 4.1901 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6704 mL | 0.838 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3352 mL | 0.419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pyridostatin is a synthetic small-molecule stabilizer of G-quadruplexe [1].
G-quadruplexe is a kind of secondary structure of DNA that usually exists in the end of the chromosome or the telomeres. Since G-quadruplexe is also enriched in the promoters of a serious of proto-oncogenes including c-kit, K-ras and Bcl-2, they are thought to participate in the regulation of gene replication and transcription. Besides that, G-quadruplexe has been found to affect the elongation, replication and capping of telomeres. Based on these findings, a lot of small molecules that can interact with G-quadruplexe have been designed and synthesized to help demonstrate the existence and roles of G-quadruplexe or to be developed as selective anti-cancer drugs. It has been reported that some small molecules interacting with G-quadruplexe can cause the progressive shortening of telomeres and subsequently the active the DNA damage response resulting in cell cycle arrest. Among these molecules, pyridostatin is a synthetic small-molecule stabilizer of G-quadruplexe with the ability to adapt the dynamic and diverse structures of G-quadruplex. Pyridostatin competed for binding with the telomere associated proteins and induced the dysfunction of telomeres [1 and 2].
In the FRET melting assay using human telomeric G-quadruplex-forming sequence and ds-DNA, pyridostatin showed maximal stabilization effect of the G-quadruplex sequence at concentration of 1 μM while showed no effect on the ds-DNA. In a panel of three cancer cell lines (HeLa, U2OS and HT1080) and a normal cell line (WI-38), treatment of pyridostatin significantly inhibited cell growth with IC50 values of 0.89 to 10 μM after 72 hours. The selectivity of pyridostatin against HT1080 cells was 18-fold higher than that against the normal cells [1 and 3].
References:
[1] Mela I, Kranaster R, Henderson R M, et al. Demonstration of ligand decoration, and ligand-induced perturbation, of G-quadruplexes in a plasmid using atomic force microscopy. Biochemistry, 2012, 51(2): 578-585.
[2] Müller S, Sanders D A, Di Antonio M, et al. Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells. Organic & biomolecular chemistry, 2012, 10(32): 6537-6546.
[3] McLuckie K I E, Di Antonio M, Zecchini H, et al. G-quadruplex DNA as a molecular target for induced synthetic lethality in cancer cells. Journal of the American Chemical Society, 2013, 135(26): 9640-9643.
- 23S-hydroxy-11,15-dioxo-ganoderic acid DM
Catalog No.:BCN8131
CAS No.:1085273-49-9
- Eupahualin C
Catalog No.:BCN7234
CAS No.:108525-39-9
- Ilexgenin A
Catalog No.:BCC9233
CAS No.:108524-94-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- Noradrenaline bitartrate monohydrate
Catalog No.:BCC4810
CAS No.:108341-18-0
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- Lumichrome
Catalog No.:BCN7083
CAS No.:1086-80-2
-
4-Hydroxy-Teriflunomide
Catalog No.:BCC4734
CAS No.:
- GSK2126458
Catalog No.:BCC3884
CAS No.:1086062-66-9
- Mizolastine
Catalog No.:BCC4521
CAS No.:108612-45-9
- Purpureaside C
Catalog No.:BCN3865
CAS No.:108648-07-3
- Eupalinolide K
Catalog No.:BCN6199
CAS No.:108657-10-9
- Y 11
Catalog No.:BCC6206
CAS No.:1086639-59-9
- 1-O-Acetyl-6beta-O-Isobutyrylbritannilactone
Catalog No.:BCN1628
CAS No.:1087072-50-1
- Isomeranzin
Catalog No.:BCN5882
CAS No.:1088-17-1
- Nemorubicin
Catalog No.:BCC4151
CAS No.:108852-90-0
- Dendrophenol
Catalog No.:BCC8165
CAS No.:108853-14-1
- Gardenolic acid B
Catalog No.:BCN7140
CAS No.:108864-53-5
Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells.[Pubmed:22790277]
Org Biomol Chem. 2012 Aug 28;10(32):6537-46.
The synthesis, biophysical and biological evaluation of a series of G-quadruplex interacting small molecules based on a N,N'-bis(quinolinyl)pyridine-2,6-dicarboxamide scaffold is described. The synthetic analogues were evaluated for their ability to stabilize telomeric G-quadruplex DNA, some of which showed very high stabilization potential associated with high selectivity over double-stranded DNA. The compounds exhibited growth arrest of cancer cells with detectable selectivity over normal cells. Long-time growth arrest was accompanied by senescence, where telomeric dysfunction is a predominant mechanism together with the accumulation of restricted DNA damage sites in the genome. Our data emphasize the potential of a senescence-mediated anticancer therapy through the use of G-quadruplex targeting small molecules based on the molecular framework of Pyridostatin.
Stabilization of G-quadruplex DNA and inhibition of Bcl-2 expression by a pyridostatin analog.[Pubmed:26923693]
Bioorg Med Chem Lett. 2016 Apr 1;26(7):1660-3.
The G-quadruplexes located in the P1 promoter of B-cell lymphoma-2 (Bcl-2) gene are implicated to regulate Bcl-2 expression. Here, we designed a new Pyridostatin analog named PDF, which exhibited high specificity and stabilizing effect toward G-quadruplexes. The luciferase assay demonstrated that PDF could significantly suppress Bcl-2 transcriptional activation in human laryngeal squamous carcinoma cells (Hep-2) cells. Besides, PDF also induced cell apoptosis in vitro assays. These results provide an excellent G-quadruplex specific ligand as an efficient Bcl-2 inhibitor. These results also implicate that PDF may be a potential anticancer drug to head neck cancer.


