PimaricinAntifungal macrolide polyene CAS# 7681-93-8 |
- MK-5172 hydrate
Catalog No.:BCC1763
CAS No.:1350462-55-3
- MK-5172 sodium salt
Catalog No.:BCC1765
CAS No.:1425038-27-2
- Telaprevir (VX-950)
Catalog No.:BCC2107
CAS No.:402957-28-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Danoprevir (RG7227)
Catalog No.:BCC2106
CAS No.:850876-88-9
- Vaniprevir
Catalog No.:BCC2030
CAS No.:923590-37-8
Quality Control & MSDS
Number of papers citing our products

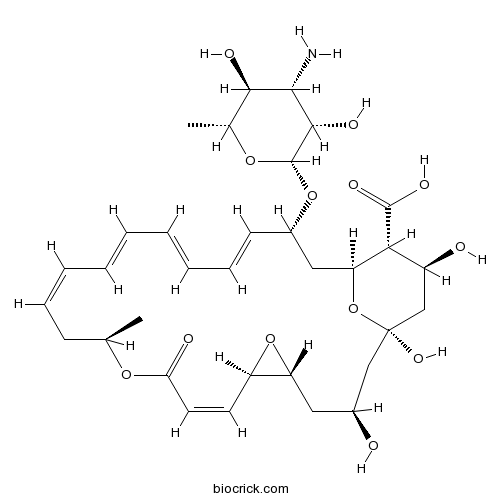
Chemical structure

3D structure
| Cas No. | 7681-93-8 | SDF | Download SDF |
| PubChem ID | 5284447 | Appearance | Powder |
| Formula | C33H47NO13 | M.Wt | 665.73 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (25.04 mM; ultrasonic and adjust pH to 6 with HCl) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-22-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid | ||
| SMILES | CC1CC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(CC3C(O3)C=CC(=O)O1)O)O)O)C(=O)O)OC4C(C(C(C(O4)C)O)N)O | ||
| Standard InChIKey | NCXMLFZGDNKEPB-FFPOYIOWSA-N | ||
| Standard InChI | InChI=1S/C33H47NO13/c1-18-10-8-6-4-3-5-7-9-11-21(45-32-30(39)28(34)29(38)19(2)44-32)15-25-27(31(40)41)22(36)17-33(42,47-25)16-20(35)14-24-23(46-24)12-13-26(37)43-18/h3-9,11-13,18-25,27-30,32,35-36,38-39,42H,10,14-17,34H2,1-2H3,(H,40,41)/b4-3+,7-5+,8-6+,11-9+,13-12+/t18-,19-,20+,21+,22+,23-,24-,25+,27-,28+,29-,30+,32+,33-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pimaricin is an effective, broad spectrum antifungal, it also exhibits reduced (oral and topical) toxicity to humans, which is probably associated with the lack of effects on mammalian cell membranes. |
| Targets | NADPH-oxidase | Antifection |
| In vivo | Genome-wide analysis of the regulation of pimaricin production in Streptomyces natalensis by reactive oxygen species.[Pubmed: 24413916]Appl Microbiol Biotechnol. 2014 Mar;98(5):2231-41.To investigate the molecular mechanisms that interplay between oxygen metabolism and secondary metabolism in Streptomyces natalensis, we compared the transcriptomes of the strains CAM.02 (ΔsodF), Pimaricin under-producer phenotype, and CAM.04 (ΔahpCD), Pimaricin over-producer phenotype, with that of the wild type at late exponential and stationary growth phases.
The negligible effects of the antifungal natamycin on cholesterol-dipalmitoyl phosphatidylcholine monolayers may explain its low oral and topical toxicity for mammals.[Pubmed: 25048356]Colloids Surf B Biointerfaces. 2014 Oct 1;122:202-8.Natamycin is an effective, broad spectrum antifungal with no reported resistance, in contrast to most antimicrobials. It also exhibits reduced (oral and topical) toxicity to humans, which is probably associated with the lack of effects on mammalian cell membranes.
|
| Animal Research | Safety and efficacy of intrastromal injection of 5% natamycin in experimental fusarium keratitis.[Pubmed: 24919100]J Ocul Pharmacol Ther. 2014 Sep;30(7):543-7.To compare the efficacy of combined intrastromal injection and topical natamycin 5% to standard topical therapy alone in an experimental rabbit model of Fusarium keratitis.
|

Pimaricin Dilution Calculator

Pimaricin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5021 mL | 7.5106 mL | 15.0211 mL | 30.0422 mL | 37.5528 mL |
| 5 mM | 0.3004 mL | 1.5021 mL | 3.0042 mL | 6.0084 mL | 7.5106 mL |
| 10 mM | 0.1502 mL | 0.7511 mL | 1.5021 mL | 3.0042 mL | 3.7553 mL |
| 50 mM | 0.03 mL | 0.1502 mL | 0.3004 mL | 0.6008 mL | 0.7511 mL |
| 100 mM | 0.015 mL | 0.0751 mL | 0.1502 mL | 0.3004 mL | 0.3755 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pimaricin, Streptomyces chattanoogensis is an antifungal macrolide polyene that binds to cell membrane sterols. Macrolides belong to a group of antibiotics that bind to the 50S ribosomal subunit and inhibit protein synthesis. Investigations show that the
- Ronidazole
Catalog No.:BCC4840
CAS No.:7681-76-7
- Potassium Iodide
Catalog No.:BCC4826
CAS No.:7681-11-0
- Azathramycin
Catalog No.:BCC1392
CAS No.:76801-85-9
- 3-Hydroxy-4',5,7-trimethoxyflavanone
Catalog No.:BCN4316
CAS No.:76792-94-4
- 2'-Hydroxydaidzein
Catalog No.:BCN4585
CAS No.:7678-85-5
- GBR 12935
Catalog No.:BCC5381
CAS No.:76778-22-8
- Lupalbigenin
Catalog No.:BCN4314
CAS No.:76754-24-0
- Pyronaridine Tetraphosphate
Catalog No.:BCC1144
CAS No.:76748-86-2
- Decumbenine
Catalog No.:BCC8312
CAS No.:76733-83-0
- Mallorepine
Catalog No.:BCN4317
CAS No.:767-98-6
- 1-Deacetylnimbolinin B
Catalog No.:BCN4313
CAS No.:76689-98-0
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- Danshensu
Catalog No.:BCN8513
CAS No.:76822-21-4
- Famotidine
Catalog No.:BCC4529
CAS No.:76824-35-6
- 5-BDBD
Catalog No.:BCC7717
CAS No.:768404-03-1
- Przewaquinone A
Catalog No.:BCN3004
CAS No.:76843-23-7
- 6-Hydroxywogonin
Catalog No.:BCN6556
CAS No.:76844-70-7
- 8-Methyl-8-azabicyclo[3.2.1]octane-3,6-diol, 9CI; (3RS,6RS)-form, 3-O-Ac
Catalog No.:BCN1361
CAS No.:7688-76-8
- Olivil 4'-O-glucoside
Catalog No.:BCN7557
CAS No.:76880-93-8
- Camptothecin
Catalog No.:BCN4318
CAS No.:7689-03-4
- Bz-Gly-OH.HCl
Catalog No.:BCC2945
CAS No.:7689-50-1
- Triacsin C
Catalog No.:BCC7377
CAS No.:76896-80-5
- 13-Methyl-8,11,13-podocarpatriene-3,12-diol
Catalog No.:BCN1360
CAS No.:769140-74-1
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Safety and efficacy of intrastromal injection of 5% natamycin in experimental fusarium keratitis.[Pubmed:24919100]
J Ocul Pharmacol Ther. 2014 Sep;30(7):543-7.
PURPOSE: To compare the efficacy of combined intrastromal injection and topical natamycin 5% to standard topical therapy alone in an experimental rabbit model of Fusarium keratitis. METHODS: Fungal keratitis was induced in the right eyes of 12 New Zealand rabbits by stromal injection of Fusarium solani spore suspension into the cornea. Four days after inoculation, animals were randomly assigned to 2 different treatment groups (n=6 in each group). The study group received intrastromal injections of natamycin 5% on treatment day 1 and 4, combined with topical natamycin 5% eye drops given hourly between 8:00 and 20:00 for the first 2 days, followed by 4 times daily on days 3-11. The control group received only topical natamycin 5% at identical intervals. Eyes were examined clinically on days 1, 4, 7, and 11 for status of corneal healing, corneal vascularization, and hypopyon. Animals were sacrificed on day 11, and corneas were subjected to histopathological examination. RESULTS: Both groups showed significant improvement in terms of conjunctival hyperemia, size and density of corneal infiltrate, corneal edema, and total clinical score. In the study group, there was a significant improvement in the height of hypopyon in the anterior chamber, while there was also an increased amount of vascularization. CONCLUSIONS: This study showed that intrastromal injection of natamycin 5% combined with topical treatment has little beneficial effect over topical therapy in a Fusarium keratitis rabbit model. The addition of intrastromal injection should be reserved to the most severe or recalcitrant cases.
The negligible effects of the antifungal natamycin on cholesterol-dipalmitoyl phosphatidylcholine monolayers may explain its low oral and topical toxicity for mammals.[Pubmed:25048356]
Colloids Surf B Biointerfaces. 2014 Oct 1;122:202-208.
Natamycin is an effective, broad spectrum antifungal with no reported resistance, in contrast to most antimicrobials. It also exhibits reduced (oral and topical) toxicity to humans, which is probably associated with the lack of effects on mammalian cell membranes. In this paper we employ Langmuir monolayers to mimic a cell membrane, whose properties are interrogated with various techniques. We found that natamycin has negligible effects on Langmuir monolayers of dipalmitoyl phosphatidylcholine (DPPC), but it strongly affects cholesterol monolayers. Natamycin causes the surface pressure isotherm of a cholesterol monolayer to expand even at high surface pressures since it penetrates into the hydrophobic chains. It also reduces the compressibility modulus, probably because natamycin disturbs the organization of the cholesterol molecules, as inferred with polarization-modulated infrared reflection absorption spectroscopy (PM-IRRAS). In mixed cholesterol/DPPC monolayers, strong effects from natamycin were only observed when the cholesterol concentration was 50mol% or higher, well above its concentration in a mammalian cell membrane. For a sterol concentration that mimics a real cell membrane in mammals, i.e. with 25mol% of cholesterol, the effects were negligible, which may explain why natamycin has low toxicity when ingested and/or employed to treat superficial fungal infections.
Genome-wide analysis of the regulation of pimaricin production in Streptomyces natalensis by reactive oxygen species.[Pubmed:24413916]
Appl Microbiol Biotechnol. 2014 Mar;98(5):2231-41.
To investigate the molecular mechanisms that interplay between oxygen metabolism and secondary metabolism in Streptomyces natalensis, we compared the transcriptomes of the strains CAM.02 (DeltasodF), Pimaricin under-producer phenotype, and CAM.04 (DeltaahpCD), Pimaricin over-producer phenotype, with that of the wild type at late exponential and stationary growth phases. Microarray data interpretation was supported by characterization of the mutant strains regarding enzymatic activities, phosphate uptake, oxygen consumption and Pimaricin production.Both mutant strains presented a delay in the transcription activation of the PhoRP system and Pimaricin biosynthetic gene cluster that correlated with the delayed inorganic phosphate (Pi) depletion in the medium and late onset of Pimaricin production, respectively. The carbon flux of both mutants was also altered: a re-direction from glycolysis to the pentose phosphate pathway (PPP) in early exponential phase followed by a transcriptional activation of both pathways in subsequent growth phases was observed. Mutant behavior diverged at the respiratory chain/tricarboxylic acid cycle (TCA) and the branched chain amino acid (BCAA) metabolism. CAM.02 (DeltasodF) presented an impaired TCA cycle and an inhibition of the BCAA biosynthesis and degradation pathways. Conversely, CAM.04 (DeltaahpCD) presented a global activation of BCAA metabolism.The results highlight the cellular NADPH/NADH ratio and the availability of biosynthetic precursors via the BCAA metabolism as the main Pimaricin biosynthetic bottlenecks under oxidative stress conditions. Furthermore, new evidences are provided regarding a crosstalk between phosphate metabolism and oxidative stress in Streptomyces.


