PF-04217903 methanesulfonateC-Met inhibitor,selective and ATP-competitive CAS# 956906-93-7 |
- MK-8033
Catalog No.:BCC1768
CAS No.:1001917-37-8
- AMG-208
Catalog No.:BCC1054
CAS No.:1002304-34-8
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- AMG-458
Catalog No.:BCC3721
CAS No.:913376-83-7
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- JNJ-38877605
Catalog No.:BCC2485
CAS No.:943540-75-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 956906-93-7 | SDF | Download SDF |
| PubChem ID | 24852079 | Appearance | Powder |
| Formula | C20H20N8O4S | M.Wt | 468.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (106.73 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | methanesulfonic acid;2-[4-[3-(quinolin-6-ylmethyl)triazolo[4,5-b]pyrazin-5-yl]pyrazol-1-yl]ethanol | ||
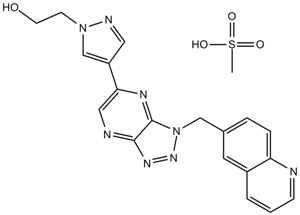
| SMILES | CS(=O)(=O)O.C1=CC2=C(C=CC(=C2)CN3C4=NC(=CN=C4N=N3)C5=CN(N=C5)CCO)N=C1 | ||
| Standard InChIKey | HBEMHKVWZJTVOC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H16N8O.CH4O3S/c28-7-6-26-12-15(9-22-26)17-10-21-18-19(23-17)27(25-24-18)11-13-3-4-16-14(8-13)2-1-5-20-16;1-5(2,3)4/h1-5,8-10,12,28H,6-7,11H2;1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective, high affinity MET inhibitor (Ki = 6-7 nM against wild type c-Met). Displays >1000-fold selectivity for c-Met over a panel of 208 kinases. |

PF-04217903 methanesulfonate Dilution Calculator

PF-04217903 methanesulfonate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1345 mL | 10.6726 mL | 21.3452 mL | 42.6903 mL | 53.3629 mL |
| 5 mM | 0.4269 mL | 2.1345 mL | 4.269 mL | 8.5381 mL | 10.6726 mL |
| 10 mM | 0.2135 mL | 1.0673 mL | 2.1345 mL | 4.269 mL | 5.3363 mL |
| 50 mM | 0.0427 mL | 0.2135 mL | 0.4269 mL | 0.8538 mL | 1.0673 mL |
| 100 mM | 0.0213 mL | 0.1067 mL | 0.2135 mL | 0.4269 mL | 0.5336 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-04217903 is an ATP-competitive small-molecule inhibitor of c-Met kinase with Ki value of 4.8 nM [1].
The c-Met kinase is a kind of receptor tyrosine kinases (RTK) and plays critical roles in embryonic development and wound healing. Activation of c-Met by the exclusive ligand hepatocyte growth factor (HGF) triggers a serious of biological responses that collectively give rise to the invasive growth .In cancers, abnormal activation of c-METs correlates with tumor growth, formation of new blood vessels and subsequently poor prognosis. PF-04217903 is a highly selective inhibitor of c-Met. It showed antitumor activity in tumor models where c-Met is activated by mechanisms including c-Met gene amplification, HGF/c-Met autocrine loop formation or c-Met overexpression [1].
PF-04217903 showed more than 1000-fold greater selectivity against c-Met kinase over 150 other kinases. When evaluated in a panel of human tumor and endothelial cell lines such as GTL-16, H1993 and HT29 cells, PF-04217903 showed inhibition of c-Met with a mean IC50 value of 7.3 nM. PF-04217903 was also found to inhibit some mutant c-Met including R988C (IC50 value of 6.4 nM), V1092I (IC50 value of 16 nM), H1094R (IC50 value of 3.1 nM), M1250T (IC50 value of 24 nM) and T11010I (IC50 value of 6.7 nM). Besides that, PF-04217903 suppressed proliferation of c-Met-amplified GTL-16 and H1993cells with IC50 values of 12 and 30 nM, respectively. It induced apoptosis in GTL-16 cells [1 and 2].
In mice bearing injected GTL-16 tumors, administration of PF-04217903 showed dose-dependent c-Met phosphorylation inhibition and antitumor efficacy. It inhibited the phosphorylation of c-Met with EC50 value of 10 nM and suppressed tumor growth with EC50 value of 13 nM. Moreover, PF-04217903 was found to affect the downstream signal transduction of c-Met such as AKT, STAT5 and Gab-1 [1].
References:
[1] Zou H Y, Li Q, Lee J H, et al. Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Molecular cancer therapeutics, 2012, 11(4): 1036-1047.
[2] Cui J J, McTigue M, Nambu M, et al. Discovery of a Novel Class of Exquisitely Selective Mesenchymal-Epithelial Transition Factor (c-MET) Protein Kinase Inhibitors and Identification of the Clinical Candidate 2-(4-(1-(Quinolin-6-ylmethyl)-1 H-[1, 2, 3] triazolo [4, 5-b] pyrazin-6-yl)-1 H-pyrazol-1-yl) ethanol (PF-04217903) for the Treatment of Cancer. Journal of medicinal chemistry, 2012, 55(18): 8091-8109.
- PF-04217903
Catalog No.:BCC2486
CAS No.:956905-27-4
- Betamethasone hydrochloride
Catalog No.:BCC4256
CAS No.:956901-32-9
- Euscaphin B
Catalog No.:BCN4507
CAS No.:956869-95-7
- 8beta,9alpha-Dihydroxylindan-4(5),7(11)-dien-8alpha,12-olide
Catalog No.:BCN8024
CAS No.:956707-04-3
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
- MM-22
Catalog No.:BCC6114
CAS No.:956605-71-3
- UNBS 5162
Catalog No.:BCC4008
CAS No.:956590-23-1
- Demethylsonchifolin
Catalog No.:BCN4551
CAS No.:956384-55-7
- Ranolazine 2HCl
Catalog No.:BCC2503
CAS No.:95635-56-6
- Ranolazine
Catalog No.:BCC3847
CAS No.:95635-55-5
- Phoyunnanin C
Catalog No.:BCN3686
CAS No.:956344-38-0
- mavatrep
Catalog No.:BCC6457
CAS No.:956274-94-5
- XL147
Catalog No.:BCC2487
CAS No.:956958-53-5
- Isolinderalactone
Catalog No.:BCN1252
CAS No.:957-66-4
- 7-Aminocephalosporanic acid
Catalog No.:BCC4617
CAS No.:957-68-6
- GDC-0941
Catalog No.:BCC3626
CAS No.:957054-30-7
- GDC-0941 dimethanesulfonate
Catalog No.:BCC1590
CAS No.:957054-33-0
- MK-3207 HCl
Catalog No.:BCC4420
CAS No.:957116-20-0
- MK-3207
Catalog No.:BCC1759
CAS No.:957118-49-9
- SM-164
Catalog No.:BCC4002
CAS No.:957135-43-2
- NVP-QAV-572
Catalog No.:BCC4181
CAS No.:957209-68-6
- BTZ043 Racemate
Catalog No.:BCC2488
CAS No.:957217-65-1
- Hedyotisol A
Catalog No.:BCN4508
CAS No.:95732-59-5
- Daphnodorin B
Catalog No.:BCN7937
CAS No.:95733-02-1
Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-MET) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1 -yl)ethanol (PF-04217903) for the treatment of cancer.[Pubmed:22924734]
J Med Chem. 2012 Sep 27;55(18):8091-109.
The c-MET receptor tyrosine kinase is an attractive oncology target because of its critical role in human oncogenesis and tumor progression. An oxindole hydrazide hit 6 was identified during a c-MET HTS campaign and subsequently demonstrated to have an unusual degree of selectivity against a broad array of other kinases. The cocrystal structure of the related oxindole hydrazide c-MET inhibitor 10 with a nonphosphorylated c-MET kinase domain revealed a unique binding mode associated with the exquisite selectivity profile. The chemically labile oxindole hydrazide scaffold was replaced with a chemically and metabolically stable triazolopyrazine scaffold using structure based drug design. Medicinal chemistry lead optimization produced 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1 -yl)ethanol (2, PF-04217903), an extremely potent and exquisitely selective c-MET inhibitor. 2 demonstrated effective tumor growth inhibition in c-MET dependent tumor models with good oral PK properties and an acceptable safety profile in preclinical studies. 2 progressed to clinical evaluation in a Phase I oncology setting.
Discovery of small molecule c-Met inhibitors: Evolution and profiles of clinical candidates.[Pubmed:20015007]
Anticancer Agents Med Chem. 2010 Jan;10(1):7-27.
The scatter factor/hepatocyte growth factor (HGF)-c-Met axis is involved in the malignant phenotype of various tumor types via activation of a wide range of autocrine and paracrine processes. Autocrine activation of tumor cell c-Met receptors enhances tumor cell proliferation, angiogenesis, invasion/metastasis and resistance to apoptosis and cytotoxic therapies. In addition, tumor and stroma cell-derived HGF functions as a potent angiogenic factor. Therefore, the HGF-c-Met axis is critically involved in multiple facets of normal cellular growth and homeostasis and activated in a dysregulated manner in a variety of cancers. Consequently, inhibiting the HGF-c-Met axis would be anticipated to have potent anti-tumor effects in many cancers through multiple complimentary mechanisms including increased sensitivity to current cytotoxic chemo-and radiotherapies. The acceptance of c-Met as a tractable target for cancer therapy has fostered intensive drug discovery efforts across the pharmaceutical industry. This research has led to 20 published crystal structures (with and without ligands) that revealed two distinct binding modes for ATP-competitive inhibitors: Type I ligands which assumes a U shape geometry through interactions with both hinge and activation loop residue Y1230, and Type II ligands which adopt a more extended orientation, either binding a conventional DFG-out conformation or protein conformations with varying degrees of 'DFG-out' character. Nearly a dozen small molecule c-Met inhibitors have entered human clinical trials ranging from Type I inhibitors solely selective for c-Met to Type I inhibitors with broader kinase activities to Type II inhibitors with "spectrum selective" kinase activity. The identification, profiles and properties of these clinical candidates are summarized in this review.
Novel therapeutic inhibitors of the c-Met signaling pathway in cancer.[Pubmed:19318488]
Clin Cancer Res. 2009 Apr 1;15(7):2207-14.
A wide variety of human malignancies exhibit sustained c-Met stimulation, overexpression, or mutation, including carcinomas of the breast, liver, lung, ovary, kidney, and thyroid. Notably, activating mutations in c-Met have been positively identified in patients with a particular hereditary form of papillary renal cancer, directly implicating c-Met in human tumorigenesis. Aberrant signaling of the c-Met signaling pathway due to dysregulation of the c-Met receptor or overexpression of its ligand, hepatocyte growth factor (HGF), has been associated with an aggressive phenotype. Extensive evidence that c-Met signaling is involved in the progression and spread of several cancers and an enhanced understanding of its role in disease have generated considerable interest in c-Met and HGF as major targets in cancer drug development. This has led to the development of a variety of c-Met pathway antagonists with potential clinical applications. The three main approaches of pathway-selective anticancer drug development have included antagonism of ligand/receptor interaction, inhibition of the tyrosine kinase catalytic activity, and blockade of the receptor/effector interaction. Several c-Met antagonists are now under clinical investigation. Preliminary clinical results of several of these agents, including both monoclonal antibodies and small-molecule tyrosine kinase inhibitors, have been encouraging. Several multitargeted therapies have also been under investigation in the clinic and have demonstrated promise, particularly with regard to tyrosine kinase inhibition.
Enzymatic characterization of c-Met receptor tyrosine kinase oncogenic mutants and kinetic studies with aminopyridine and triazolopyrazine inhibitors.[Pubmed:19459657]
Biochemistry. 2009 Jun 16;48(23):5339-49.
The c-Met receptor tyrosine kinase (RTK) is a key regulator in cancer, in part, through oncogenic mutations. Eight clinically relevant mutants were characterized by biochemical, biophysical, and cellular methods. The c-Met catalytic domain was highly active in the unphosphorylated state (k(cat) = 1.0 s(-1)) and achieved 160-fold enhanced catalytic efficiency (k(cat)/K(m)) upon activation to 425000 s(-1) M(-1). c-Met mutants had 2-10-fold higher basal enzymatic activity (k(cat)) but achieved maximal activities similar to those of wild-type c-Met, except for Y1235D, which underwent a reduction in maximal activity. Small enhancements of basal activity were shown to have profound effects on the acquisition of full enzymatic activity achieved through accelerating rates of autophosphorylation. Biophysical analysis of c-Met mutants revealed minimal melting temperature differences indicating that the mutations did not alter protein stability. A model of RTK activation is proposed to describe how a RTK response may be matched to a biological context through enzymatic properties. Two c-Met clinical candidates from aminopyridine and triazolopyrazine chemical series (PF-02341066 and PF-04217903) were studied. Biochemically, each series produced molecules that are highly selective against a large panel of kinases, with PF-04217903 (>1000-fold selective relative to 208 kinases) being more selective than PF-02341066. Although these prototype inhibitors have similar potencies against wild-type c-Met (K(i) = 6-7 nM), significant differences in potency were observed for clinically relevant mutations evaluated in both biochemical and cellular contexts. In particular, PF-02341066 was 180-fold more active against the Y1230C mutant c-Met than PF-04217903. These highly optimized inhibitors indicate that for kinases susceptible to active site mutations, inhibitor design may need to balance overall kinase selectivity with the ability to inhibit multiple mutant forms of the kinase (penetrance).


