PB-22Synthetic cannabinoid CAS# 1400742-17-7 |
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- Nepicastat
Catalog No.:BCC1795
CAS No.:173997-05-2
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- CP-945598 HCl
Catalog No.:BCC1082
CAS No.:686347-12-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1400742-17-7 | SDF | Download SDF |
| PubChem ID | 71604304 | Appearance | Powder |
| Formula | C23H22N2O2 | M.Wt | 358.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO | ||
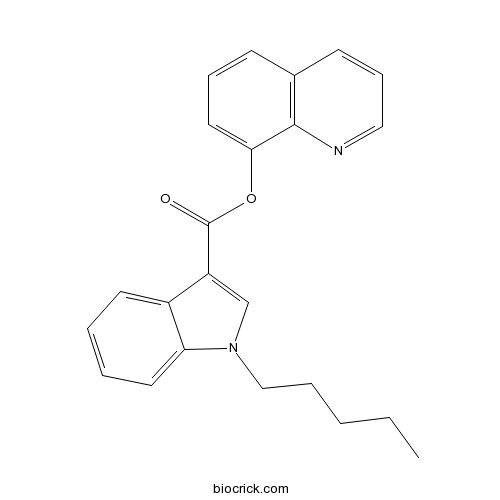
| Chemical Name | quinolin-8-yl 1-pentylindole-3-carboxylate | ||
| SMILES | CCCCCN1C=C(C2=CC=CC=C21)C(=O)OC3=CC=CC4=C3N=CC=C4 | ||
| Standard InChIKey | ZAVGICCEAOUWFM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H22N2O2/c1-2-3-6-15-25-16-19(18-11-4-5-12-20(18)25)23(26)27-21-13-7-9-17-10-8-14-24-22(17)21/h4-5,7-14,16H,2-3,6,15H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

PB-22 Dilution Calculator

PB-22 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7899 mL | 13.9497 mL | 27.8995 mL | 55.7989 mL | 69.7486 mL |
| 5 mM | 0.558 mL | 2.7899 mL | 5.5799 mL | 11.1598 mL | 13.9497 mL |
| 10 mM | 0.279 mL | 1.395 mL | 2.7899 mL | 5.5799 mL | 6.9749 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.116 mL | 1.395 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: N/A PB-22 or 1-pentyl-1H-indole-3-carboxylic acid 8-quinolinyl ester is a designer drug offered by online vendors as a cannabimimetic agent, and detected being sold in synthetic cannabis products in Japan in 2013. The physiological and toxicological properties of this compound are not known. PB-22 is now found in many herbal incense and potpourri products [1]. in vitro: N/A in vivo: N/A Clinical trial: N/A
- Eucalyptin acetate
Catalog No.:BCN6216
CAS No.:14004-35-4
- 4μ8C
Catalog No.:BCC4754
CAS No.:14003-96-4
- Nystatin (Fungicidin)
Catalog No.:BCC4813
CAS No.:1400-61-9
- Dimethylolurea
Catalog No.:BCC8943
CAS No.:140-95-4
- 4-Allylanisole
Catalog No.:BCC8674
CAS No.:140-67-0
- Pentamidine isethionate
Catalog No.:BCC5644
CAS No.:140-64-7
- Nithiamide
Catalog No.:BCC4687
CAS No.:140-40-9
- Cinnamic acid
Catalog No.:BCN6217
CAS No.:140-10-3
- Palbinone
Catalog No.:BCN3930
CAS No.:139954-00-0
- Alpinone 3-acetate
Catalog No.:BCN7768
CAS No.:139906-49-3
- Drahebenine
Catalog No.:BCN7044
CAS No.:1399049-43-4
- Globularin
Catalog No.:BCN6215
CAS No.:1399-49-1
- 3-CPMT
Catalog No.:BCC6845
CAS No.:14008-79-8
- Tannic acid
Catalog No.:BCN2643
CAS No.:1401-55-4
- AMG 925
Catalog No.:BCC5150
CAS No.:1401033-86-0
- 3'-Hydroxygynuramide II
Catalog No.:BCC8634
CAS No.:1401093-57-9
- PI-1840
Catalog No.:BCC5453
CAS No.:1401223-22-0
- ML 277
Catalog No.:BCC7976
CAS No.:1401242-74-7
- 15,16-Dihydroxyoctadeca-9Z,12Z-dienoic acid
Catalog No.:BCC8438
CAS No.:140129-22-2
- Pterisolic acid A
Catalog No.:BCN4842
CAS No.:1401419-85-9
- Pterisolic acid B
Catalog No.:BCN4843
CAS No.:1401419-86-0
- Pterisolic acid C
Catalog No.:BCN4838
CAS No.:1401419-87-1
- Pterisolic acid D
Catalog No.:BCN4839
CAS No.:1401419-88-2
- Pterisolic acid E
Catalog No.:BCN4841
CAS No.:1401419-89-3
Schedules of Controlled Substances: Extension of Temporary Placement of PB-22, 5F-PB-22, AB-FUBINACA and ADB-PINACA in Schedule I of the Controlled Substances Act. Final order.[Pubmed:26859904]
Fed Regist. 2016 Feb 5;81(24):6175-7.
The Administrator of the Drug Enforcement Administration is issuing this final order to extend the temporary schedule I status of four synthetic cannabinoids pursuant to the temporary scheduling provisions of the Controlled Substances Act. The substances are: quinolin-8-yl 1-pentyl-1H-indole-3-carboxylate (PB-22; QUPIC); quinolin-8-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate (5-fluoro-PB-22; 5F-PB-22); N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA); and N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (ADB-PINACA), including their optical, positional and geometric isomers, salts, and salts of isomers. The current final order temporarily placing PB-22, 5F-PB-22, AB-FUBINACA, and ADB-PINACA into schedule I is in effect through February 9, 2016. This final order will extend the temporary scheduling of PB-22, 5F-PB-22, AB-FUBINACA, and ADB-PINACA for one year, or until the permanent scheduling action for these four substances is completed, whichever occurs first.
Analytical differentiation of quinolinyl- and isoquinolinyl-substituted 1-(5-fluoropentyl)-1H-indole-3-carboxylates: 5F-PB-22 and its ten isomers.[Pubmed:28127408]
Forensic Toxicol. 2017;35(1):56-65.
Differentiation among regioisomers of synthetic cannabinoids in forensic drug analysis is a crucial issue, since all isomers are not regulated by law. New equivalent analogs obtained via minor modification of their preexisting molecules keep on emerging. Isomers formed via substitutional exchange are also a cause for concern. This study is focused on the isomeric molecules that stem from minor modifications of 5F-PB-22. The analytical properties of these molecules and methods of differentiation are reported. Scan mode analysis using gas chromatography-electron ionization-mass spectrometry (GC-EI-MS) was performed using the authentic 5F-PB-22 standard, five regioisomeric quinolinyl ester indoles, and five regioisomeric isoquinolinyl ester indoles. Because it was not possible to separate 5F-PB-22 from the 5-hydroxyquinoline isomer using GC and all analytes showed similar EI mass spectra, liquid chromatography (LC)-tandem mass spectrometry analysis was performed. Using LC, a successful separation of 5F-PB-22 from all isomers could be achieved. Based on the electrospray ionization-mass spectra, the protonated molecular ion at m/z 377.2 was selected as the precursor ion for the regioisomeric and structural isomeric differentiation. Collision-induced dissociation provides relative intensity differences in the product ions among the isomers, enabling mass spectrometric differentiation of the isomers. To our knowledge, this is the first report on mass spectrometric differentiation of 5F-PB-22 and its ten isomers.
In Vitro and In Vivo Human Metabolism of Synthetic Cannabinoids FDU-PB-22 and FUB-PB-22.[Pubmed:26810398]
AAPS J. 2016 Mar;18(2):455-64.
In 2014, FDU-PB-22 and FUB-PB-22, two novel synthetic cannabinoids, were detected in herbal blends in Japan, Russia, and Germany and were quickly added to their scheduled drugs list. Unfortunately, no human metabolism data are currently available, making it challenging to confirm their intake. The present study aims to identify appropriate analytical markers by investigating FDU-PB-22 and FUB-PB-22 metabolism in human hepatocytes and confirm the results in authentic urine specimens. For metabolic stability, 1 muM FDU-PB-22 and FUB-PB-22 was incubated with human liver microsomes for up to 1 h; for metabolite profiling, 10 muM was incubated with human hepatocytes for 3 h. Two authentic urine specimens from FDU-PB-22 and FUB-PB-22 positive cases were analyzed after beta-glucuronidase hydrolysis. Metabolite identification in hepatocyte samples and urine specimens was accomplished by high-resolution mass spectrometry using information-dependent acquisition. Both FDU-PB-22 and FUB-PB-22 were rapidly metabolized in HLM with half-lives of 12.4 and 11.5 min, respectively. In human hepatocyte samples, we identified seven metabolites for both compounds, generated by ester hydrolysis and further hydroxylation and/or glucuronidation. After ester hydrolysis, FDU-PB-22 and FUB-PB-22 yielded the same metabolite M7, fluorobenzylindole-3-carboxylic acid (FBI-COOH). M7 and M6 (hydroxylated FBI-COOH) were the major metabolites. In authentic urine specimens after beta-glucuronidase hydrolysis, M6 and M7 also were the predominant metabolites. Based on our study, we recommend M6 (hydroxylated FBI-COOH) and M7 (FBI-COOH) as suitable urinary markers for documenting FDU-PB-22 and/or FUB-PB-22 intake.
Schedules of Controlled Substances: Placement of PB-22, 5F-PB-22, AB-FUBINACA and ADB-PINACA into Schedule I. Final rule.[Pubmed:27632803]
Fed Regist. 2016 Sep 6;81(172):61130-3.
With the issuance of this final rule, the Drug Enforcement Administration places quinolin-8-yl 1-pentyl-1H-indole-3-carboxylate (PB-22; QUPIC), quinolin-8-yl 1-(5-fluoropentyl)-1H-indole-3-carboxylate (5-fluoro-PB-22; 5F-PB-22), N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) and N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (ADB-PINACA), including their salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible, into schedule I of the Controlled Substances Act. This scheduling action is pursuant to the Controlled Substances Act which requires that such actions be made on the record after opportunity for a hearing through formal rulemaking. This action imposes the regulatory controls and administrative, civil, and criminal sanctions applicable to schedule I controlled substances on persons who handle (manufacture, distribute, reverse distribute, import, export, engage in research, conduct instructional activities or chemical analysis, or possess), or propose to handle PB-22, 5F-PB-22, AB-FUBINACA, or ADB-PINACA.


