MPDCCAS# 159262-32-5 |
- XL-888
Catalog No.:BCC2339
CAS No.:1149705-71-4
- 17-DMAG (Alvespimycin) HCl
Catalog No.:BCC1175
CAS No.:467214-21-7
- BIIB021
Catalog No.:BCC2124
CAS No.:848695-25-0
- IPI-504 (Retaspimycin hydrochloride)
Catalog No.:BCC2126
CAS No.:857402-63-2
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- PF-04929113 (SNX-5422)
Catalog No.:BCC2130
CAS No.:908115-27-5
Quality Control & MSDS
Number of papers citing our products

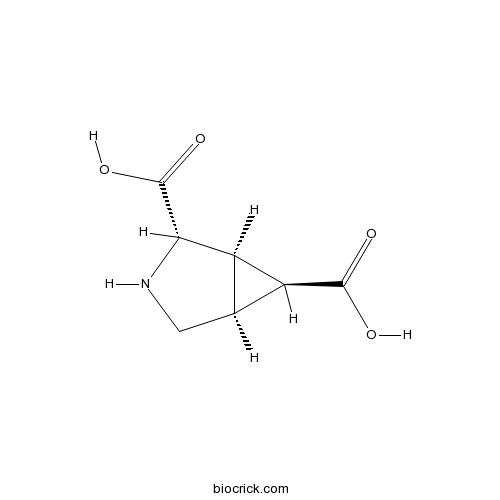
Chemical structure

3D structure
| Cas No. | 159262-32-5 | SDF | Download SDF |
| PubChem ID | 6604785 | Appearance | Powder |
| Formula | C7H9NO4 | M.Wt | 171.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (1R,2S,5S,6S)-3-azabicyclo[3.1.0]hexane-2,6-dicarboxylic acid | ||
| SMILES | C1C2C(C2C(=O)O)C(N1)C(=O)O | ||
| Standard InChIKey | UNNFLFDQCHJXPI-QTBDOELSSA-N | ||
| Standard InChI | InChI=1S/C7H9NO4/c9-6(10)4-2-1-8-5(3(2)4)7(11)12/h2-5,8H,1H2,(H,9,10)(H,11,12)/t2-,3+,4-,5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of the Na+-dependent high-affinity synaptosomal glutamate transporter. |

MPDC Dilution Calculator

MPDC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8428 mL | 29.2141 mL | 58.4283 mL | 116.8566 mL | 146.0707 mL |
| 5 mM | 1.1686 mL | 5.8428 mL | 11.6857 mL | 23.3713 mL | 29.2141 mL |
| 10 mM | 0.5843 mL | 2.9214 mL | 5.8428 mL | 11.6857 mL | 14.6071 mL |
| 50 mM | 0.1169 mL | 0.5843 mL | 1.1686 mL | 2.3371 mL | 2.9214 mL |
| 100 mM | 0.0584 mL | 0.2921 mL | 0.5843 mL | 1.1686 mL | 1.4607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PNU 22394 hydrochloride
Catalog No.:BCC7285
CAS No.:15923-42-9
- Isokaempferide
Catalog No.:BCN3790
CAS No.:1592-70-7
- L-NIO dihydrochloride
Catalog No.:BCC6689
CAS No.:159190-44-0
- MM 77 dihydrochloride
Catalog No.:BCC6854
CAS No.:159187-70-9
- L-755,507
Catalog No.:BCC7282
CAS No.:159182-43-1
- CARIPORIDE
Catalog No.:BCC6432
CAS No.:159138-80-4
- 3F8
Catalog No.:BCC6112
CAS No.:159109-11-2
- F1839-I
Catalog No.:BCN6450
CAS No.:159096-49-8
- 6-Benzyloxyindole
Catalog No.:BCC8769
CAS No.:15903-94-3
- Wedelobatin B
Catalog No.:BCN6730
CAS No.:1589488-35-6
- Wedelobatin A
Catalog No.:BCN6731
CAS No.:1589488-34-5
- Secoisolarisiresinol Diglucoside
Catalog No.:BCC9140
CAS No.:158932-33-3
- Everolimus (RAD001)
Catalog No.:BCC3594
CAS No.:159351-69-6
- N-Acetyl-O-phosphono-Tyr-Glu-Glu-Ile-Glu
Catalog No.:BCC5853
CAS No.:159439-02-8
- Enfuvirtide
Catalog No.:BCC5641
CAS No.:159519-65-0
- Fluconazole mesylate
Catalog No.:BCC4236
CAS No.:159532-41-9
- GR 55562 dihydrochloride
Catalog No.:BCC6913
CAS No.:159533-25-2
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
- Saropyrone
Catalog No.:BCN7692
CAS No.:159650-12-1
- Wikstrol A
Catalog No.:BCN7938
CAS No.:159736-35-3
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
- Ibutamoren Mesylate
Catalog No.:BCC1638
CAS No.:159752-10-0
- Fmoc-Lys(Ac)-OH
Catalog No.:BCC3514
CAS No.:159766-56-0
Evaluation of cell damage induced by irradiated Zinc-Phthalocyanine-gold dendrimeric nanoparticles in a breast cancer cell line.[Pubmed:30348269]
Biomed J. 2018 Aug;41(4):254-264.
BACKGROUND: Cancer is a non-communicable disease that occurs following a mutation in the genes which control cell growth. Breast cancer is the most diagnosed cancer among South African women and a major cause of cancer-related deaths worldwide. Photodynamic therapy (PDT) is an alternative cancer therapy that uses photochemotherapeutic agents, known as photosensitizers. Drug-delivery nanoparticles are commonly used in nanomedicine to enhance drug-therapeutic efficiency. This study evaluated the photodynamic effects following treatment with 0.3 muM multiple particles delivery complex (MPDC) and irradiated with a laser fluence of 10 J/cm(2) using a 680 nm diode laser in a breast cancer cell line (MCF-7). METHODS: Cell damage was assessed by inverted light microscopy for cell morphology; the Apoptox-Glo triple assay was used for cell viability, caspase activity and identification of cytodamage markers; flow cytometric analysis for cell death pathways and mitochondrial membrane potential; the enzyme linked immunosorbent assay (ELISA) for cytochrome C release; and real-time reverse transcriptase polymerase chain reaction (RT-PCR) array for gene expression. RESULTS: Laser activated-MPDC induced a significant change in morphology of PDT-treated cells, with the appearance of apoptotic like morphological features. An increase in cytotoxicity, caspase activity, cell depolarization and cytochrome C release were identified in PDT-treated cells. Finally, the upregulation of BAX, BCL-2, CASP-2 and ULK-1 genes was observed. CONCLUSION: The MPDC yielded a successful and stable hybrid agent with potent photodynamic abilities.
Characterization of a multiple particle delivery complex and determination of cellular photodamage in skin fibroblast and breast cancer cell lines.[Pubmed:28715120]
J Biophotonics. 2018 Feb;11(2).
Zinc metallized Phthalocyanine (ZnPcSmix ), a potent photosensitizer, is conjugated to gold dendrimer encapsulated nanoparticles (AuDENPs) in order to improve the efficacy of photodynamic therapy (PDT) using MCF-7 breast cancer cells and WS1 fibroblast cells as a control. Both ZnPcSmix and AuDENPs are mixed in a nitrogen atmosphere for 48 hours and characterization analysis conducted using ultraviolet-visible (UV-vis) spectrometry for spectral properties, transmission electron microscopy (TEM) for morphological features and zeta potential measurement for surface stability and size distribution of the compound obtained or of the multiple particles delivery complex (MPDC). Cell viability, proliferation and membrane damage following PDT are assessed by the trypan blue exclusion test, adenosine triphosphate luminescence and lactate dehydrogenase cytotoxicity assays, respectively. Stable MPDCs are spherical shaped with a diameter lesser than 5 nm, and have a maximum absorption peak at 676 nm. The MPDC-mediated PDT induces a decrease in cell viability and proliferation, and increased membrane damage or cytotoxicity. The conjugation enhances the therapeutic efficiency of PDT by improving drug delivery and targeting of MCF-7 cancer cells.
A novel catheter-guidance algorithm for localization of atrial fibrillation rotor and focal sources.[Pubmed:28268380]
Conf Proc IEEE Eng Med Biol Soc. 2016 Aug;2016:501-504.
Locating atrial fibrillation (AF) focal and rotor sources can help improve target ablation therapy for AF. However, it remains unclear how to use the information provided by multi-polar diagnostic catheters (MPDC) to locate AF sources. Our aim was to develop a catheter-guidance algorithm to locate AF focal and rotor sources using a conventional MPDC. We simulated a 10 cm x 10 cm atrial tissue with focal and rotor sources using the Nygren et al. ionic model. We modeled a Lasso MPDC with 20-unipole electrodes placed with a spacing of 4.5-1-4.5 mm (diameter, d=15 mm) along a circle to obtain 10-bipole electrograms. Staring from an initial location, the algorithm, which was blinded to the location and type of the AF source, iteratively advanced the MPDC by moving its center to the location of the first activated bipole (FAB). The algorithm located an AF source if a stopping condition for either source was satisfied using bipole electrogram characteristics extracted from the MPDC placement. We tested the algorithm for a single rotor and focal source for all possible initial positions on the simulated tissue and repeated it for a random placement with a maximum of 20 possible placements at every trial. The algorithm located the AF source for 100% of trials and on average required 5.99 +/- 1.92 placements to an AF source. This algorithm may be used to iteratively direct an MPDC towards an AF source and allow the AF source to be localized for customized AF ablation.
Characterization of Electrograms from Multipolar Diagnostic Catheters during Atrial Fibrillation.[Pubmed:26581316]
Biomed Res Int. 2015;2015:272954.
Atrial fibrillation (AF) is the most common arrhythmia in USA with more than 2.3 million people affected annually. Catheter ablation procedure is a method for treatment of AF, which involves 3D electroanatomic mapping of the patient's left atrium (LA) by maneuvering a conventional multipolar diagnostic catheter (MPDC) along the LA endocardial surface after which pulmonary vein (PV) isolation is performed, thus eliminating the AF triggers originating from the PVs. However, it remains unclear how to effectively utilize the information provided by the MPDC to locate the AF-sustaining sites, known as sustained rotor-like activities (RotAs). In this study, we use computer modeling to investigate the variations in the characteristics of the MPDC electrograms, namely, total conduction delay (TCD) and average cycle length (CL), as the MPDC moves towards a RotA source. Subsequently, a study with a human subject was performed in order to verify the predictions of the simulation study. The conclusions from this study may be used to iteratively direct an MPDC towards RotA sources thus allowing the RotAs to be localized for customized and improved AF ablation.
No more tears? Maternal involvement during the newborn screening examination.[Pubmed:21540279]
Clin Pediatr (Phila). 2011 Aug;50(8):753-6.
BACKGROUND: Babies often show signs of discomfort and distress by crying during the neonatal screening examination (NSE). The authors hypothesized that supporting the baby with maternal participation may reduce infant crying during NSE. The objective of this study was to document incidental infant crying during NSE, before and after training residents, on maternal involvement and infant comfort techniques to help. METHODS: A total of 20 NSEs of normal newborn babies by pediatric residents were observed (video-recorded) following informed consent of the doctor and the baby's mother. The examining doctors were then taught how to use maternal participation and developmental care (MPDC) comfort techniques to support the baby during NSE. Mothers were shown how to focus on their baby's needs by supporting the baby's head (preventing atonic neck reflexes) and, if necessary, providing nonnutritive sucking to the baby and an encouraging, repetitive low-tone voices to sooth the baby. A further 14 NSEs on different babies were video-recorded using these techniques. The video recordings were analyzed by independent observers for total length of crying and duration of crying during specific components of the NSE. Mothers in both groups were given a questionnaire to assess their opinions of the NSE. RESULTS: The median length of crying was significantly longer in the pre-MPDC group (93.5 seconds; range 0-198 seconds) compared with the post-MPDC infants (0 seconds; range 0-123 seconds; P = .001). Only 1 of 20 infants in the pre-MPDC did not cry during NSE compared with 8 of 14 babies in the post-MPDC group. CONCLUSION: Newborn infants cry less and mothers were more satisfied with NSEs when shown simple support and comfort techniques for their babies.
The role of cation binding in determining substrate selectivity of glutamate transporters.[Pubmed:19074430]
J Biol Chem. 2009 Feb 13;284(7):4510-5.
Glutamate transport is coupled to the co-transport of 3Na(+) and 1H(+) and the countertransport of 1 K(+). However, the mechanism of how this process occurs is not well understood. The crystal structure of an archaeal homolog of the human glutamate transporters, Glt(Ph), has provided the framework to begin to understand the mechanism of transport. The glutamate transporter EAAT2 is different from other subtypes in two respects. First, Li(+) cannot support transport by EAAT2, whereas it can support transport by the other excitatory amino acid transporters, and second, EAAT2 is sensitive to a wider range of blockers than other subtypes. We have investigated the relationship between the cation driving transport and whether the glutamate analogues, l-anti-endo-3,4-methanopyrrolidine-dicarboxylic acid (MPDC) and (2S,4R)-4-methylglutamate (4MG), are substrates or blockers of transport. We have also investigated the molecular basis for these differences. EAAT2 has a Ser residue at position 441 with hairpin loop 2, whereas the corresponding residue in EAAT1 is a Gly residue. We demonstrate that if the transporter has a Ser residue at this position, then 4MG and MPDC are poor substrates in Na(+), and Li(+) cannot support transport of any substrate. Conversely, if the transporter has a Gly residue at this position, then in Na(+) 4MG and MPDC are substrates with efficacy comparable with glutamate, but in Li(+) 4MG and MPDC are poor substrates relative to glutamate. This Ser/Gly residue is located between the bound substrate and one of the cation binding sites, which provides an explanation for the coupling of substrate and cation binding.
Bi(III)4-methylpiperidinedithiocarbamate coprecipitation procedure for separation--pre-concentration of trace metal ions in water samples by flame atomic absorption spectrometric determination.[Pubmed:17467895]
J Hazard Mater. 2007 Oct 1;149(1):160-5.
A pre-concentration method was developed for determination of trace amounts of cadmium, copper and lead in water samples by FAAS after coprecipitation by using potassium 4-methylpiperidinedithiocarbamate (K4-MPDC) as a chelating agent and Bi(III) as a carrier element. This procedure is based on filtration of the solution containing precipitate on a cellulose nitrate membrane filter following Cd(II), Cu(II) and Pb(II) coprecipitation with Bi(III)4-MPDC and then the precipitates together with membrane filter were dissolved in concentrated nitric acid. The metal contents of the final solution were determined by FAAS. Several parameters including pH of sample solution, amount of carrier element and reagent, standing time, sample volume for precipitation and the effects of diverse ions were examined. The accuracy of the method was tested with standard reference material (MBH, C31XB20 and CRM BCR-32) and Cd, Cu and Pb added samples. Determination of Cd, Cu and Pb was carried out in sea water, river water and tap water samples. The recoveries were >95%. The relative standard deviations of determination were less than 10%.
A conformationally constrained competitive inhibitor of the sodium-dependent glutamate transporter in forebrain synaptosomes: L-anti-endo-3,4-methanopyrrolidine dicarboxylate.[Pubmed:7970177]
Neurosci Lett. 1994 Jun 20;174(2):193-7.
A series of L-3,4-methanopyrrolidine dicarboxylate isomers were investigated as potential inhibitors of the high affinity, sodium-dependent glutamate transporter in rat forebrain synaptosomes. Of the isomers tested, only L-anti-endo-3,4-methanopyrrolidine dicarboxylate (L-anti-endo-MPDC) blocked the uptake of [3H]D-aspartate, a non-metabolized substrate. Kinetic analysis demonstrated that L-anti-endo-MPDC is a potent competitive inhibitor (Ki = 5 microM) comparable to that of L-glutamate and L-trans-2,4-pyrrolidine dicarboxylate (L-trans-2,4-PDC). Conformational analysis of L-glutamate, L-trans-2,4-PDC and L-anti-endo-MPDC are used to refine the pharmacophore model of the transporter binding site.


