MNSInhibitor of Src/Syk tyrosine kinases CAS# 1485-00-3 |
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- Firategrast
Catalog No.:BCC1575
CAS No.:402567-16-2
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
Quality Control & MSDS
Number of papers citing our products

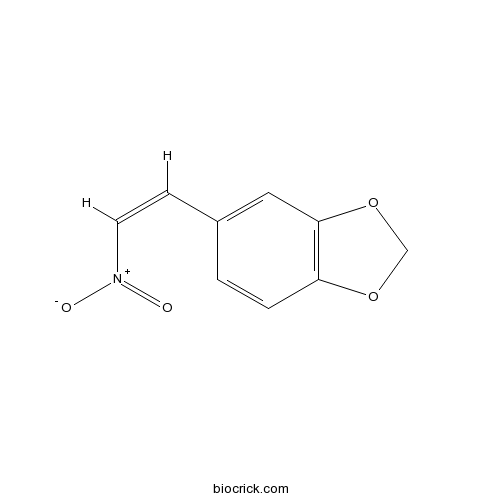
Chemical structure
3D structure
| Cas No. | 1485-00-3 | SDF | Download SDF |
| PubChem ID | 5354438 | Appearance | Powder |
| Formula | C9H7NO4 | M.Wt | 193.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 170724; 5-(2-Nitrovinyl)benzodioxole | ||
| Solubility | DMSO : 50 mg/mL (258.85 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 5-[(Z)-2-nitroethenyl]-1,3-benzodioxole | ||
| SMILES | C1OC2=C(O1)C=C(C=C2)C=C[N+](=O)[O-] | ||
| Standard InChIKey | KFLWBZPSJQPRDD-ARJAWSKDSA-N | ||
| Standard InChI | InChI=1S/C9H7NO4/c11-10(12)4-3-7-1-2-8-9(5-7)14-6-13-8/h1-5H,6H2/b4-3- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of Src and Syk tyrosine kinases. Displays antiaggregative activity via inhibition of GPIIb/IIIa activation (IC50 = 12.7 μM for thrombin-induced platelet aggregation). Exhibits no effects on Ca2+-dependent enzymes, PKC or arachidonic acid metabolism. |

MNS Dilution Calculator

MNS Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1771 mL | 25.8853 mL | 51.7706 mL | 103.5411 mL | 129.4264 mL |
| 5 mM | 1.0354 mL | 5.1771 mL | 10.3541 mL | 20.7082 mL | 25.8853 mL |
| 10 mM | 0.5177 mL | 2.5885 mL | 5.1771 mL | 10.3541 mL | 12.9426 mL |
| 50 mM | 0.1035 mL | 0.5177 mL | 1.0354 mL | 2.0708 mL | 2.5885 mL |
| 100 mM | 0.0518 mL | 0.2589 mL | 0.5177 mL | 1.0354 mL | 1.2943 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective inhibitor of Src and Syk tyrosine kinases. Displays antiaggregative activity via inhibition of GPIIb/IIIa activation (IC50 = 12.7 μM for thrombin-induced platelet aggregation). Exhibits no effects on Ca2+-dependent enzymes, PKC or arachidonic ac
- JMV 390-1
Catalog No.:BCC5922
CAS No.:148473-36-3
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- Fmoc-Lys(Dnp)-OH
Catalog No.:BCC3519
CAS No.:148083-64-1
- GRK2i
Catalog No.:BCC6048
CAS No.:148505-03-7
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
One-pot synthesis of gamma-MnS/reduced graphene oxide with enhanced performance for aqueous asymmetric supercapacitors.[Pubmed:28050971]
Nanotechnology. 2017 Feb 10;28(6):065402.
In this work, gamma-MNS/reduced graphene oxide composites (gamma-MNS/rGO) were prepared using a facile one-pot hydrothermal method. As an electrode material for supercapacitors, the gamma-MNS/rGO-60 composite obtained under dosages of graphene oxide was 60 mg and exhibited an enhanced specific capacitance of 547.6 F g(-1) at a current density of 1 A g(-1), and outstanding rate capability (65% capacitance retention at 20 A g(-1)), with superior cycling stability and electrochemical reversibility. An asymmetric supercapacitor assembled from gamma-MNS/rGO-60 composite and rGO (gamma-MNS/rGO-60//rGO) showed a voltage window of 0-1.6 V and delivered a high energy density of 23.1 W h kg(-1) at a power density of 798.8 W kg(-1), and 15.9 W h kg(-1) at 4.5 kW kg(-1). Moreover, two such 1.0 x 1.0 cm(2) devices connected together in series easily light up a group of LED lights, showing its potential practical application as an attractive energy storage device.
Dual emissions from MnS clusters confined in the sodalite nanocage of a chalcogenide-based semiconductor zeolite.[Pubmed:28265630]
Dalton Trans. 2017 Mar 21;46(12):3929-3933.
A new host-guest hybrid system with MNS clusters confined in a chalcogenide-based semiconductor zeolite was for the first time constructed and its photoluminescence (PL) properties were also investigated. The existence of MNS clusters in the nanopores of the semiconductor zeolite was revealed by UV-Vis absorption spectroscopy, steady-state fluorescence analysis, Raman as well as Fourier transform infrared (FTIR) spectroscopy. The aggregation state of the entrapped MNS clusters at different measurement temperatures was probed by electron paramagnetic resonance (EPR) spectroscopy. Of significant importance is the fact that the entrapped MNS clusters displayed dual emissions at 518 nm (2.39 eV) and 746 nm (1.66 eV), respectively, and the long-wavelength emission has never been observed in other MNS-confined host-guest systems. These two emission peaks displayed tunable PL intensity affected by the loading level and measurement temperature. This can be explained by the different morphologies of MNS clusters with different aggregation states at the corresponding loading level or measurement temperature. The current study opens a new avenue to construct inorganic chalcogenide cluster involved host-guest systems with a semiconductor zeolite as the host matrix.
MNS, Duffy, and Kell blood groups among the Uygur population of Xinjiang, China.[Pubmed:28301670]
Genet Mol Res. 2017 Mar 15;16(1). pii: gmr-16-01-gmr.16019176.
Human blood groups are a significant resource for patients, leading to a fierce international competition in the screening of rare blood groups. Some rare blood group screening programs have been implemented in western countries and Japan, but not particularly in China. Recently, the genetic background of ABO and Rh blood groups for different ethnic groups or regions in China has been focused on increasingly. However, rare blood groups such as MN, Duffy, Kidd, MNS, and Diego are largely unexplored. No systematic reports exist concerning the polymorphisms and allele frequencies of rare blood groups in China's ethnic minorities such as Uygur and Kazak populations of Xinjiang, unlike those on the Han population. Therefore, this study aimed to investigate the allele frequencies of rare blood groups, namely, MNS, Duffy, Kell, Dombrock, Diego, Kidd, Scianna, Colton, and Lutheran in the Uygur population of Xinjiang Single specific primer-polymerase chain reaction was performed for genotyping and statistical analysis of 9 rare blood groups in 158 Uygur individuals. Allele frequencies were compared with distribution among other ethnic groups. Observed and expected values of genotype frequencies were compared using the chi-square test. Genotype frequencies obeyed the Hardy-Weinberg equilibrium (P > 0.5) and allele frequencies were stable. Of all subjects detected, 4 cases carried the rare phenotype S(-)s(-) of MNS blood group (frequency of 0.0253), and 1 case carried the phenotype Jk(a-b-) (frequency of 0.0063). Frequencies of the four groups, MNS, Duffy, Dombrock, and Diego, in the Uygur population differed from those in other ethnic groups. Gene distribution of the Kell, Kidd, and Colton was similar to that in Tibetan and Han populations, though there were some discrepancies. Gene distribution of Scianna and Lutheran groups showed monomorphism similar to that in Tibetan and Han populations. These findings could contribute to the investigation of the origin, evolution, and hematology of Uygur population of Xinjiang and assist in screening of rare blood groups in ethnic minorities, meeting of clinical blood supply demands, and building of the national rare blood group library.
The distribution of MNS hybrid glycophorins with Mur antigen expression in Chinese donors including identification of a novel GYP.Bun allele.[Pubmed:27232276]
Vox Sang. 2016 Oct;111(3):308-314.
BACKGROUND AND OBJECTIVES: MNS hybrid glycophorins are identified by characteristic antigen profiles. One of these is the Mur antigen, which is expressed on red cell hybrid glycophorins of several phenotypes of the 'Miltenberger' series found predominantly in East Asian population. The aim of this study was to investigate the distribution of Mur-positive hybrid glycophorins and clarify the genetic basis in the donors from southern China. MATERIALS AND METHODS: Blood samples from 528 donors were collected for Mur antigen serological typing. Sequencing of GYPB pseudoexon 3 and MNS phenotyping were conducted in Mur-positive samples. The multiplex ligation-dependent probe amplification (MLPA) was used to confirm the zygosity of the GYP.Mur allele and determine the MNSs genotype. The expression of Mur antigen was evaluated by flow cytometry. RESULTS: Fifty-one Mur-positive samples were identified by serological testing. Sequencing analysis showed 50 donors (50/528, 9.5%) with the GYP.Mur allele (48 heterozygotes and two homozygotes), which were confirmed by the MLPA genotyping analysis, and one donor (1/528, 0.19%) with a novel GYP.Bun allele. Flow cytometry analysis revealed higher Mur antigen expression on GP.Mur (Mi.III) homozygotes than heterozygotes. For the GYP.Mur homozygotes, an incorrect 'N' positive typing with anti-N lectin was obtained. CONCLUSION: GP.Mur (Mi.III) is the main Mur-positive hybrid glycophorin in Guangzhou donors. The dosage effect of Mur antigen observed provides a basis for selecting the homozygous GP.Mur RBCs as the reagent cells to avoid neglecting weak antibodies. A separate GYP.Bun lineage found in the southern China provides evidence for further complexity in the MNS system.
Synthesis and pharmacological evaluation of novel beta-nitrostyrene derivatives as tyrosine kinase inhibitors with potent antiplatelet activity.[Pubmed:17601492]
Biochem Pharmacol. 2007 Aug 15;74(4):601-11.
Protein tyrosine kinases have been known to be involved in regulation of platelet aggregation, suggesting a potential target for antiplatelet therapy. Our previous study showed that 3,4-methylenedioxy-beta-nitrostyrene (MNS) prevented platelet aggregation caused by various stimulators, and this action was accompanied by inhibition of tyrosine kinases. In the present study, in order to examine the structural determinants required for the actions of MNS and to develop more potent tyrosine kinase inhibitors and antiplatelet agents, a new series of beta-nitrostyrene derivatives were synthesized and pharmacologically characterized. The beta-nitrostyrene derivatives inhibited thrombin- or collagen-induced human platelet aggregation, ATP secretion, GPIIb/IIIa activation and protein tyrosine phosphorylation. In recombinant enzyme assay, some beta-nitrostyrene derivatives also demonstrated potent inhibition of Src and/or Syk kinase activity. Furthermore, there was a good correlation between the inhibitory potency of these compounds on tyrosine kinases and on platelet activation/aggregation. Among them, a benzoyl ester derivative (compound 10) possess up to 8-fold greater potency than MNS and over two orders of magnitude greater potency than genistein or tyrphostin A47 in inhibiting platelet responses to thrombin. Our data suggest that beta-nitrostyrenes may represent a new class of tyrosine kinase inhibitors with potent antiplatelet activity.
Prevention of platelet glycoprotein IIb/IIIa activation by 3,4-methylenedioxy-beta-nitrostyrene, a novel tyrosine kinase inhibitor.[Pubmed:16837624]
Mol Pharmacol. 2006 Oct;70(4):1380-9.
Binding fibrinogen to activated glycoprotein (GP)IIb/IIIa is the final common pathway of platelet aggregation and has become a successful target for antiplatelet therapy. In the present study, we found that a small chemical compound, 3,4-methyl-enedioxy-beta-nitrostyrene (MNS), exhibited potent and broad-spectrum inhibitory effects on human platelet aggregation caused by various stimulators. Moreover, addition of MNS to human platelets that had been aggregated by ADP caused a rapid disaggregation. We demonstrated that the antiaggregatory activity of MNS is due to inhibition of GPIIb/IIIa activation by measuring the binding amount of PAC-1 in platelets. In contrast, MNS is not a direct antagonist of GPIIb/IIIa, because MNS did not affect fibrinogen binding to fixed ADP-stimulated platelets. By investigating how MNS inhibits GPIIb/IIIa activation, we found that MNS potently inhibited the activity of tyrosine kinases (Src and Syk) and prevented protein tyrosine phosphorylation and cytoskeletal association of GPIIb/IIIa and talin, but it had no direct effects on protein kinase C, Ca2+ mobilization, Ca2+-dependent enzymes (myosin light chain kinase and calpain), and arachidonic acid metabolism, and it did not affect the cellular levels of cyclic nucleotides. Therefore, MNS represents a new class of tyrosine kinase inhibitor that potently prevents GPIIb/IIIa activation and platelet aggregation without directly affecting other signaling pathways required for platelet activation. Because MNS inhibits GPIIb/IIIa functions in a manner different from GPIIb/IIIa antagonists, this feature may provide a new strategy for treatment of platelet-dependent thrombosis.


