MG-132Proteasome inhibitor, Cell permeable, reversible CAS# 133407-82-6 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 133407-82-6 | SDF | Download SDF |
| PubChem ID | 107707 | Appearance | Powder |
| Formula | C26H41N3O5 | M.Wt | 475.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MG132,Z-LLL-al,Z-Leu-Leu-Leu-CHO | ||
| Solubility | DMSO : 83.33 mg/mL (175.20 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
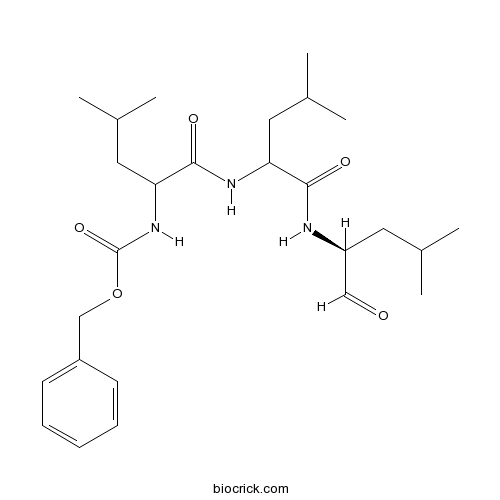
| Chemical Name | benzyl N-[4-methyl-1-[[4-methyl-1-[[(2S)-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]carbamate | ||
| SMILES | CC(C)CC(C=O)NC(=O)C(CC(C)C)NC(=O)C(CC(C)C)NC(=O)OCC1=CC=CC=C1 | ||
| Standard InChIKey | TZYWCYJVHRLUCT-UVKLAMSESA-N | ||
| Standard InChI | InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22?,23?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent cell-permeable inhibitor of proteasome (IC50 = 100 nM) and calpain (IC50 = 1.2 μM). Inhibits TNF-α-induced NF-κB activation and IκBα degradation. Induces neurite outgrowth in PC12 cells and has anticancer properties in vitro. |

MG-132 Dilution Calculator

MG-132 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1026 mL | 10.513 mL | 21.0261 mL | 42.0521 mL | 52.5652 mL |
| 5 mM | 0.4205 mL | 2.1026 mL | 4.2052 mL | 8.4104 mL | 10.513 mL |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1026 mL | 4.2052 mL | 5.2565 mL |
| 50 mM | 0.0421 mL | 0.2103 mL | 0.4205 mL | 0.841 mL | 1.0513 mL |
| 100 mM | 0.021 mL | 0.1051 mL | 0.2103 mL | 0.4205 mL | 0.5257 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
MG-132 significantly increased MCPIP1 expression through a mechanism involving de novo mRNA synthesis and activated apoptosis through induction of caspases 3/7 in HepG2 and HeLa cells.
Abstract
Intraperitoneal administration of MG-132 reversed several pathogenic changes in OVE26 diabetic mice though up-regulation of Nrf2-mediated antioxidative function and down-regulation of NF-KB-mediated inflammation.
Abstract
The combination of MG-132 and camptothecin exhibited strongest cytotoxicity against MIA PaCa-2 pancreatic cancer cells though promoting apoptosis induced by caspase-3 that is possibly associated with up-regulation of Noxa and down-regulation of Mcl-1. However, the combination of MG-132 and doxorubicin exhibited less cytotoxicity due to decreased apoptosis correlated with high levels of Mcl-1.
Abstract
Administration of DJ-1 protein in rats pre-treated with MG-132 resulted in the reduction of α-synuclein mRNA, hypoxia-inducible factor 1α mRNA and α-synuclein protein.
Abstract
MG-132, a peptidyl-aldehyde proteasome inhibitor, induced differentiation of PC12 cells that was accompanied by phosphorylation of TrkA, prolonged activation of Src and activation of ERK1/2 with nuclear translocation of Src.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MG132 (carbobenzoxy-Leu-Leu-leucinal) as a peptide aldehyde effectively blocks the proteolytic activity of proteasome complex.9 Proteasome inhibitors including MG132 have been shown to induce apoptotic cell death through formation of ROS. ROS formation and GSH depletion due to proteasome inhibitors may cause mitochondrial dysfunction and subsequent cytochrome c release, which leads to cell viability loss1, 2.
MG132 dose dependently inhibited the growth of A549 cells with an IC50 of approximately 20 µM. MG132 also reduced the growth of human cervical HeLa cancer cells with an IC50 of approximately 5 µM. Treatment with 0.5 µM MG132 significantly decreased the growth of HeLa cells and induced cell death as well. Cell growth inhibition by MG132 depends on incubation doses of that and cell types3.
MG132 significantly induced a G1 phase arrest of the cell cycle. It inhibits the growth of HT-29 colon cancer cells via inducing G2/M cell cycle arrest4, causes MG-63 osteosarcoma cell arrest at G2/M phase5, prolongs the duration of G0/G1 arrest in MnCl2-treated A549 cells21 and induces a G1 arrest in gastric carcinoma cells6. Deregulation of the ubiquitin-proteasomal system by MG132 can result in different cell cycle phase arrests depending on various cancer cell lines.
Proteasome inhibitors including MG132 have been shown to induce apoptotic cell death through formation of ROS1, 2, 7. MG132 inhibited the growth of human A549 cells via inducing the cell cycle arrest as well as triggering apoptosis, which was in part correlated with the changes of ROS and GSH levels.
References:
1. Ling YH, Liebes L, Zou Y and Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells, 2003; 278: 33714–33723.
2. Qiu JH, Asai A, Chi S, et al. Proteasome inhibitors induce cytochrome c-caspase-3-like protease-mediated apoptosis in cultured cortical neurons. J Neurosci 2000; 20: 259–265.
3. YH. Han, WH. Park, MG132 as a proteasome inhibitor induces cell growth inhibition and cell death in A549 lung cancer cells via influencing reactive oxygen species and GSH level, Human and Experimental Toxicology, 29(7) 607–614.
4. Wu WK, Wu YC, Yu L, et al. Induction of autophagy by proteasome inhibitor is associated with proliferative arrest in colon cancer cells. Biochem Biophys Res Commun 2008; 374: 258–263.
5. Yan XB, Yang DS, Gao X, et al. Caspase-8 dependent osteosarcoma cell apoptosis induced by proteasome inhibitor MG132. Cell Biol Int 2007; 31: 1136–1143.
6. ZhangW, Tong Q, Li S, Wang X andWang Q.MG-132 inhibits telomerase activity, induces apoptosis and G(1) arrest associated with upregulated p27kip1 expression and downregulated survivin expression in gastric carcinoma cells. Cancer Invest 2008; 26:1032–1036.
7. Wu HM, Chi KH, Lin WW. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett 2002; 526: 101–105.
- (+)-Aflavazole
Catalog No.:BCN7339
CAS No.:133401-09-9
- 3-MPPI
Catalog No.:BCC6705
CAS No.:133399-65-2
- Brandioside
Catalog No.:BCN6770
CAS No.:133393-81-4
- Fmoc-β-Homo-D-Tyr(tBu)-OH
Catalog No.:BCC2620
CAS No.:133373-24-7
- 4-Hydroxy-11,12,13-trinor-5-eudesmen-7-one
Catalog No.:BCN6627
CAS No.:133369-42-3
- Fmoc-His(MMt)-OH
Catalog No.:BCC3503
CAS No.:133367-33-6
- DV 7028 hydrochloride
Catalog No.:BCC6202
CAS No.:133364-62-2
- Daturataturin A
Catalog No.:BCN6179
CAS No.:133360-51-7
- Lydicamycin
Catalog No.:BCN1843
CAS No.:133352-27-9
- Lactacystin
Catalog No.:BCN1841
CAS No.:133343-34-7
- CHR-6494
Catalog No.:BCC1479
CAS No.:1333377-65-3
- Imbricataflavone A
Catalog No.:BCN8025
CAS No.:133336-96-6
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- PF 1022A
Catalog No.:BCC8064
CAS No.:133413-70-4
- 3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone
Catalog No.:BCN1583
CAS No.:1334309-44-2
- Angophorol
Catalog No.:BCN3965
CAS No.:133442-54-3
- Heudelotinone
Catalog No.:BCN6180
CAS No.:133453-58-4
- Iloperidone
Catalog No.:BCC2516
CAS No.:133454-47-4
- Fmoc-Glu(OAll)-OH
Catalog No.:BCC3492
CAS No.:133464-46-7
- TAI-1
Catalog No.:BCC5576
CAS No.:1334921-03-7
- SR 1001
Catalog No.:BCC6309
CAS No.:1335106-03-0
- Exendin-3 (9-39) amide
Catalog No.:BCC7257
CAS No.:133514-43-9
- Apigenin 4'-O-rhamnoside
Catalog No.:BCN6181
CAS No.:133538-77-9
- AG-490
Catalog No.:BCC2193
CAS No.:133550-30-8
[Synergistic anti-tumor effect of obatoclax and MG-132 in esophageal cancer cell line CaES-17].[Pubmed:27113178]
Nan Fang Yi Ke Da Xue Xue Bao. 2016 Apr;36(4):506-13.
OBJECTIVE: To explore whether MG-132 could enhance the anti-tumor activity of obatoclax against esophageal cancer cell line CaES-17. METHODS: MTT assay was used to determine the cytotoxicity of obatoclax and MG-132 in CaES-17 cells. The IC(50) of obatoclax and MG-132 were used to determine the molar ratio (1:2.4) of the two drugs for combined treatment of the cells. The concentrations of obatoclax and MG-132 ranged from 1/8 IC(50) to 4 IC(50) after serial dilution, and their combination index (CI) was calculated using CompuSyn software. The expression of ubiquitin and the cleavage of PARP, caspase-9, phospho-histone H3 and phospho-aurora A/B/C in the exposed cells were examined with Western blotting; the cell apoptosis was measured by flow cytometry with Annexin V staining, and the percentage of cells in each cell cycle phase was also determined by flow cytometry. RESULTS: The CI of obatoclax and MG-132 was 0.296 for a 50% inhibition of Caes-17 cells and was 0.104 for a 95% inhibition. The cells treated with obatoclax or MG-132 alone showed increased expression of ubiquitin and cleavage of PARP and caspase-9. Compared with the cells treated with obatoclax or MG-132 alone, the cells with a combined treatment exhibited significantly increased expression of ubiquitin, cleavage of PARP and caspase-9, and expression of phospho-Histone H3 (P<0.05). The combined treatment of the cells also resulted in significantly increased expression of phospho-Aurora A/B/C compared with obatoclax treatment alone. The cells with the combined treatment showed significantly higher percentages of apoptotic cells and cells in sub-G(1) and G(2)/M phases compared with the cells treated with either of the drugs (P<0.05). CONCLUSION: Obatoclax combined with MG-132 shows a significant synergistic anti-tumor effect against esophageal cancer CaES-17 cells by inducing apoptosis and cell cycle arrest.
Proteasome inhibitor MG-132 enhances histone deacetylase inhibitor SAHA-induced cell death of chronic myeloid leukemia cells by an ROS-mediated mechanism and downregulation of the Bcr-Abl fusion protein.[Pubmed:26722260]
Oncol Lett. 2015 Nov;10(5):2899-2904.
Recently, there has been progress in the treatment of chronic myeloid leukemia (CML). However, novel therapeutic strategies are required in order to address the emerging problem of imatinib resistance. Histone deacetylase inhibitors (HDACi) and proteasome inhibitors are promising alternatives, and may be amenable to integration with current therapeutic approaches. However, the mechanisms underlying the interaction between these two agents remain unclear. The present study assessed the cytotoxic effect of the HDACi, suberoylanilide hydroxamic acid (SAHA), in combination with the proteasome inhibitor, MG-132, in imatinib-sensitive K562 and imatinib-resistant K562G cells, and investigated the mechanism underlying this effect. Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method and protein expression levels were determined by western blotting. Reactive oxygen species (ROS) generation levels were observed under a fluorescence microscope The results indicated that SAHA and MG-132 act in a synergistic manner to induce cell death in K562 and K562G cells. This effect was associated with Bcr-Abl downregulation and the production of ROS. Notably, the ROS scavenger, N-acetyl-L-cysteine, almost fully reversed the cell death and Bcr-Abl downregulation that was induced by the combination of SAHA and MG-132. By contrast, the pan-caspase inhibitor, z-VAD-fmk, only partially reversed the cell death induced by these two drugs in CML cells. These results indicated that increased intracellular ROS levels are important in the induction of cell death and the downregulation of Bcr-Abl. In conclusion, the present results suggested that combined SAHA and MG-132 may be a promising treatment for CML.
Chemically assisted somatic cell nuclear transfer without micromanipulator in the goat: effects of demecolcine, cytochalasin-B, and MG-132 on the efficiency of a manual method of oocyte enucleation using a pulled Pasteur pipette.[Pubmed:25956201]
Anim Reprod Sci. 2015 Jul;158:11-8.
The present study aimed to facilitate widespread application of a previously described manual method of somatic cell nuclear transfer (SCNT) by investigating the effects of demecolcine (a microtubule-depolymerizing chemical), cytochalasin-B (a microfilament-depolymerizing chemical: 2.5mug/ml for 15min) and MG-132 (a proteasome inhibitor chemical) on the (i) incidence of cytoplasmic protrusion of MII chromosomes, (ii) improvement of manual oocyte enucleation, and (iii) in vitro and in vivo developmental competence of SCNT embryos in the goat. Following in vitro maturation, around 65% of goat oocytes contained a characteristic cytoplasmic protrusion of MII-chromosomes. Treatment with demecolcine (0.4mug/ml for 30min) significantly increased this rate to 92.2+/-4.5%. Treatment with MG-132 (2muM for 30min) could not improve this rate when used alone (61.4+/-11.5%), but when combined with demecolcine (86.4+/-8.1%). Treatment with cytochalasin-B completely suppressed this rate whenever used, either alone (7.7+/-5.1%) or in combination with demecolcine (3.9+/-1.3%). In a direct comparison, there was no significant difference in quantity and quality of embryos propagated by the manual vs. micromanipulation-based methods of SCNT (cleavage: 85.3+/-4.5 vs. 89.5+/-8.9%, blastocyst: 19.5+/-4.3 vs. 24.3+/-4.4%, grade 1 and 2 blastocyst: 33.8+/-7.1 vs. 29.5+/-6.3%, total cell count: 125+/-11.1 vs. 122+/-10.5, respectively). Furthermore, development to live kids at term was not significant between the two SCNT methods. From both technical and economical points of view, the overall in vitro and in vivo efficiency of this manual method of SCNT proved it a simple, fast and efficient alternative for large scale production of cloned goats.
Inhibition of autophagy promotes cell apoptosis induced by the proteasome inhibitor MG-132 in human esophageal squamous cell carcinoma EC9706 cells.[Pubmed:26137056]
Oncol Lett. 2015 May;9(5):2278-2282.
Lysosome-dependent macroautophagy, also termed autophagy, and the ubiquitin-proteasome system and are the primary intracellular pathways involved in protein degradation. Previous studies have demonstrated that proteasome inhibitors are able to inhibit tumor growth and activate autophagy. The present study investigated the effect of the proteasome inhibitor MG-132 on cellular proliferation using a cell counting kit 8 assay, and the effect of the agent on apoptosis and autophagy was assessed using flow cytometry and monodansylcadaverine, respectively. Western blot analysis was used to investigate protein changes during the course of treatment. It was revealed that MG-132 inhibited cell proliferation, activated autophagy and induced cell death in EC9706 cells. Autophagy was activated through the class III PI3K pathway, and the expression of the Beclin-1 protein was determined to be significantly upregulated. However, the autophagy inhibitor 3-methyladenine (3-MA) inhibited the expression of the autophagy-associated protein Beclin-1 and reduced the accumulation of autophagic vacuoles induced by MG-132. MG-132-induced apoptosis was enhanced by the autophagy inhibitor 3-MA, which may be a result of caspase-3 activation in the EC9706 cells. These findings suggest that inhibition of the proteasome can induce autophagy in human ESCC cells, and also increase cell death. This indicates that proteasome inhibitors may be potential novel anti-cancer agents for the adjuvant treatment of esophageal squamous cell carcinoma.
Potential of the proteasomal inhibitor MG-132 as an anticancer agent, alone and in combination.[Pubmed:11911275]
Anticancer Res. 2001 Nov-Dec;21(6A):3941-7.
Proteasomal activity is required for normal cellular functions including cell division, where entry and exit from mitosis is strictly regulated by cyclins and cyclin-dependent kinases which are among the important substrates of the proteasomal degradative machinery. Inhibitors of proteasomal activity have been shown to be effective inducers of apoptosis in tumor cells and may be useful as anticancer agents, either alone or in combination with other drugs. We have examined the effect of MG-132, a dipeptide proteasomal inhibitor, on various human cancer cell lines. We have also examined the effect of MG-132 on normal CD34+ enriched primary human peripheral blood stem cells. Our results indicate that MG-312 is a potent anticancer agent with cytotoxic effects on a variety of human cancer cell lines irrespective of their p53 status. MG-132 was found to be more effective in combination with drugs such as doxorubicin and etoposide that act in the S/G2-phase of the cell cycle via a mechanism that involves stabilization of cyclin B1 and increased expression of Bax. Further, MG-132 inhibits CFU-GM colony formation of the CD34+ enriched PBSC population and this inhibition correlates with release of cyt C into the cytosol.
Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine.[Pubmed:8830056]
J Biochem. 1996 Mar;119(3):572-6.
To explore membrane-permeable synthetic inhibitors that discriminate between endogenous calpain and proteasome in cells, we examined the inhibition of profiles against calpain and proteasome in vitro and in vivo of peptidyl aldehydes possessing di-leucine and tri-leucine. The tripeptide aldehyde benzyloxycarbonyl-leucyl-leucinal (ZLLLal) strongly inhibited calpain and proteasome activities in vitro. The concentration required for 50% inhibition (IC50) of the casein-degrading activity of calpain was 1.25 microM, and the IC50s for the succinyl-leucyl-leucyl-valyl-tyrosine-4-methylcoumaryl-7-amide (Suc-LLVY-MCA)- and benzyloxycarbonyl-leucyl-leucyl-leucine-4-methylcoumaryl -7-amide (ZLLL-MCA)-degrading activities of proteasome were 850 and 100 nM, respectively. On the other hand, the synthetic dipeptide aldehyde benzyloxycarbonyl-leucyl-leucinal (ZLLal) strongly inhibited the casein degrading activity of calpain (IC50 1.20 microM), but the inhibition of proteasome was weak (IC50S for SucLLVY-MCA- and ZLLL-MCA-degrading activities were 120 and 110 microM, respectively). Thus, while calpain was inhibited by similar concentrations of ZLLal and ZLLLal, the inhibitory potencies of ZLLLal against the ZLLL-MCA- and Suc-LLVY-MCA-degrading activities in proteasome were 1,100 and 140 times stronger than those of ZLLal, respectively. To evaluate the effectiveness of these inhibitors on intracellular proteasome, the induction of neurite outgrowth in PC12 cells caused by proteasome inhibition was examined. ZLLLal and ZLLal initiated neurite outgrowth with optimal concentrations of 20 nM and 10 microM, respectively, again showing a big difference in the effective concentrations for the proteasome inhibition as in vitro. As for the effect on intracellular calpain, the concentration of ZLLLal and ZLLal required for the inhibition of the autolytic activation of calpain in rabbit erythrocytes were 100 and 100 microM or more, respectively. The almost equal inhibitory potencies of ZLLLal and ZLLal were in agreement with the inhibition of calpain in vitro. These differential effects of inhibitors against calpain and proteasome are potentially useful for identifying the functions of calpain and proteasome in cell physiology and pathology.
The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B.[Pubmed:8087845]
Cell. 1994 Sep 9;78(5):773-85.
We demonstrate an essential role for the proteasome complex in two proteolytic processes required for activation of the transcription factor NF-kappa B. The p105 precursor of the p50 subunit of NF-kappa B is processed in vitro by an ATP-dependent process that requires proteasomes and ubiquitin conjugation. The C-terminal region of p105 is rapidly degraded, leaving the N-terminal p50 domain. p105 processing can be blocked in intact cells with inhibitors of the proteasome or in yeast with proteasome mutants. These inhibitors also block the activation of NF-kappa B and the rapid degradation of I kappa B alpha induced by tumor necrosis factor alpha. Thus, the ubiquitin-proteasome pathway functions not only in the complete degradation of polypeptides, but also in the regulated processing of precursors into active proteins.


