MEK inhibitorPotent MEK inhibitor, Antitumor agent CAS# 334951-92-7 |
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- PD184352 (CI-1040)
Catalog No.:BCC1112
CAS No.:212631-79-3
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 334951-92-7 | SDF | Download SDF |
| PubChem ID | 23507698 | Appearance | Powder |
| Formula | C26H26N4O2 | M.Wt | 426.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (117.23 mM; Need ultrasonic) | ||
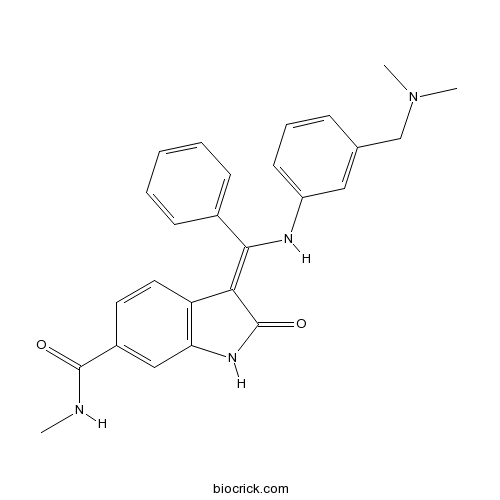
| Chemical Name | (3Z)-3-[[3-[(dimethylamino)methyl]anilino]-phenylmethylidene]-N-methyl-2-oxo-1H-indole-6-carboxamide | ||
| SMILES | CNC(=O)C1=CC2=C(C=C1)C(=C(C3=CC=CC=C3)NC4=CC=CC(=C4)CN(C)C)C(=O)N2 | ||
| Standard InChIKey | BQNHHYZMVCQLJG-VHXPQNKSSA-N | ||
| Standard InChI | InChI=1S/C26H26N4O2/c1-27-25(31)19-12-13-21-22(15-19)29-26(32)23(21)24(18-9-5-4-6-10-18)28-20-11-7-8-17(14-20)16-30(2)3/h4-15,28H,16H2,1-3H3,(H,27,31)(H,29,32)/b24-23- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MEK inhibitor is a potent MEK inhibitor with antitumor potency. |

MEK inhibitor Dilution Calculator

MEK inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3446 mL | 11.7231 mL | 23.4461 mL | 46.8922 mL | 58.6153 mL |
| 5 mM | 0.4689 mL | 2.3446 mL | 4.6892 mL | 9.3784 mL | 11.7231 mL |
| 10 mM | 0.2345 mL | 1.1723 mL | 2.3446 mL | 4.6892 mL | 5.8615 mL |
| 50 mM | 0.0469 mL | 0.2345 mL | 0.4689 mL | 0.9378 mL | 1.1723 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2345 mL | 0.4689 mL | 0.5862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A potent MEK inhibitor, Antitumor agent.
- (S)-HexylHIBO
Catalog No.:BCC7167
CAS No.:334887-48-8
- HexylHIBO
Catalog No.:BCC7166
CAS No.:334887-43-3
- Gisadenafil besylate
Catalog No.:BCC7871
CAS No.:334827-98-4
- Laburnine
Catalog No.:BCN1992
CAS No.:3348-73-0
- Tracheloside
Catalog No.:BCN2738
CAS No.:33464-71-0
- 3-Aminopiperidine dihydrochloride
Catalog No.:BCC8619
CAS No.:334618-23-4
- AH 6809
Catalog No.:BCC1332
CAS No.:33458-93-4
- Evonine
Catalog No.:BCN3087
CAS No.:33458-82-1
- Alnustone
Catalog No.:BCN2761
CAS No.:33457-62-4
- 3,3'-Bilawsone
Catalog No.:BCN7912
CAS No.:33440-64-1
- 2-Pyridylethylamine dihydrochloride
Catalog No.:BCC7379
CAS No.:3343-39-3
- Quercetin 3,4'-dimethyl ether
Catalog No.:BCN5257
CAS No.:33429-83-3
- Substance P
Catalog No.:BCC6957
CAS No.:33507-63-0
- Fucoxanthin
Catalog No.:BCN2948
CAS No.:3351-86-8
- Luteinizing Hormone Releasing Hormone (LHRH)
Catalog No.:BCC1049
CAS No.:33515-09-2
- 6'-Iodoresiniferatoxin
Catalog No.:BCC7114
CAS No.:335151-55-8
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- iMAC2
Catalog No.:BCC2396
CAS No.:335166-36-4
- Raddeanoside 20
Catalog No.:BCN2796
CAS No.:335354-79-5
- Cyclo(Phe-Leu)
Catalog No.:BCN2418
CAS No.:3354-31-2
- (-)-Bilobalide
Catalog No.:BCN1279
CAS No.:33570-04-6
- Polpunonic acid
Catalog No.:BCN7136
CAS No.:33600-93-0
- Myricanol
Catalog No.:BCN5258
CAS No.:33606-81-4
- Ispinesib (SB-715992)
Catalog No.:BCC2509
CAS No.:336113-53-2
Metastatic BRAF K601E-mutated melanoma reaches complete response to MEK inhibitor trametinib administered for over 36 months.[Pubmed:28344857]
Exp Hematol Oncol. 2017 Mar 21;6:6.
BACKGROUND: The BRAF K601E mutation occurs in 5% of patients with melanoma, and is the third most common type of BRAF mutation. However, treatment with BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors is only approved in patients with BRAF V600-positive melanoma, and patients with K601E-mutated melanoma do not have access to such drugs. CASE PRESENTATION: A female patient was diagnosed with high tumor burden metastatic melanoma harboring the BRAF K601E mutation. After chemotherapy failure, she underwent compassionate treatment with trametinib. Trametinib showed good activity and efficacy, with 48% shrinkage of a metastatic lymphadenopathy after 4 months' treatment. However, the patient reported treatment-related skin toxicity that required dosage reduction and a personalized intermittent trametinib dosing schedule. After over 36 months from the first trametinib administration, and resection of a metastatic lymphadenopathy, the patient experienced complete response. CONCLUSIONS: This case report shows that trametinib could be a valid therapeutic option in patients with metastatic melanoma harboring the rare BRAF K601E mutation.
Antidyskinetic Effects of MEK Inhibitor Are Associated with Multiple Neurochemical Alterations in the Striatum of Hemiparkinsonian Rats.[Pubmed:28337120]
Front Neurosci. 2017 Mar 9;11:112.
L-DOPA-induced dyskinesia (LID) represents one of the major problems of the long-term therapy of patients with Parkinson's disease (PD). Although, the pathophysiologic mechanisms underlying LID are not completely understood, activation of the extracellular signal regulated kinase (ERK) is recognized to play a key role. ERK is phosphorylated by mitogen-activated protein kinase kinase (MEK), and thus MEK inhibitor can prevent ERK activation. Here the effect of the MEK inhibitor PD98059 on LID and the associated molecular changes were examined. Rats with unilateral 6-OHDA lesions of the nigrostriatal pathway received daily L-DOPA treatment for 3 weeks, and abnormal involuntary movements (AIMs) were assessed every other day. PD98059 was injected in the lateral ventricle daily for 12 days starting from day 10 of L-DOPA treatment. Striatal molecular markers of LID were analyzed together with gene regulation using microarray. The administration of PD98059 significantly reduced AIMs. In addition, ERK activation and other associated molecular changes including DeltaFosB were reversed in rats treated with the MEK inhibitor. PD98059 induced significant up-regulation of 418 transcripts and down-regulation of 378 transcripts in the striatum. Tyrosine hydroxylase (Th) and aryl hydrocarbon receptor nuclear translocator (Arnt) genes were down-regulated in lesioned animals and up-regulated in L-DOPA-treated animals. Analysis of protein levels showed that PD98059 reduced the striatal TH. These results support the association of p-ERK1/2, DeltaFosB, p-H3 to the regulation of TH and ARNT in the mechanisms of LID, and pinpoint other gene regulatory changes, thus providing clues for identifying new targets for LID therapy.
A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study.[Pubmed:28339824]
Neuro Oncol. 2017 Aug 1;19(8):1135-1144.
Background: Activation of the mitogen-activated protein kinase pathway is important for growth of pediatric low-grade gliomas (LGGs). The aim of this study was to determine the recommended phase II dose (RP2D) and the dose-limiting toxicities (DLTs) of the MEK inhibitor selumetinib in children with progressive LGG. Methods: Selumetinib was administered orally starting at 33 mg/m2/dose b.i.d., using the modified continual reassessment method. Pharmacokinetic analysis was performed during the first course. BRAF aberrations in tumor tissue were determined by real-time polymerase chain reaction and fluorescence in situ hybridization. Results: Thirty-eight eligible subjects were enrolled. Dose levels 1 and 2 (33 and 43 mg/m2/dose b.i.d.) were excessively toxic. DLTs included grade 3 elevated amylase/lipase (n = 1), headache (n = 1), mucositis (n = 2), and grades 2-3 rash (n = 6). At dose level 0 (25 mg/m2/dose b.i.d, the RP2D), only 3 of 24 subjects experienced DLTs (elevated amylase/lipase, rash, and mucositis). At the R2PD, the median (range) area under the curve (AUC0-infinity) and apparent oral clearance of selumetinib were 3855 ng*h/mL (1780 to 7250 ng x h/mL) and 6.5 L x h-1 x m-2 (3.4 to 14.0 L x h-1 x m-2), respectively. Thirteen of 19 tumors had BRAF abnormalities. Among the 5 (20%) of 25 subjects with sustained partial responses, all at the RP2D, 4 had BRAF aberrations, 1 had insufficient tissue. Subjects received a median of 13 cycles (range: 1-26). Fourteen (37%) completed all protocol treatment (26 cycles [n = 13], 13 cycles [n = 1]) with at least stable disease; 2-year progression-free survival at the RP2D was 69 +/- SE 9.8%. Conclusion: Selumetinib has promising antitumor activity in children with LGG. Rash and mucositis were the most common DLTs.
Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAF(V600)-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial.[Pubmed:28268064]
Lancet Oncol. 2017 Apr;18(4):464-472.
BACKGROUND: Patients with BRAF(V600)-mutant melanoma benefit from treatment with the combination of BRAF and MEK inhibitors, but resistance and disease progression develops in most patients. Preclinical studies and case studies have indicated that acquired resistance to BRAF inhibition can be reversible. We aimed to assess the anti-tumour activity of rechallenge with BRAF plus MEK inhibition in a prospective clinical trial. METHODS: In this open-label, single arm, dual-centre, phase 2 academic study in Belgium, patients aged 18 years or older with BRAF(V600)-mutant melanoma who had previously progressed on BRAF inhibitors (with or without MEK inhibitors) and were off-treatment for at least 12 weeks, were treated with dabrafenib 150 mg orally twice per day plus trametinib 2 mg orally once per day. The primary endpoint was the proportion of patients with investigator-assessed overall response at any time (defined as complete response or partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 confirmed on two occasions, at least 28 days after the first response was recorded). Analyses were done in the intention-to-treat population. The study is ongoing but no longer recruiting patients. This trial is registered with ClinicalTrials.gov, number NCT02296996. FINDINGS: Between April 5, 2014, and Feb 2, 2016, 25 patients were enrolled and initiated treatment in our study. A partial response was documented in eight (32%) of 25 patients (95% CI 15-54; six patients had progressed on previous treatment with dabrafenib plus trametinib and two patients had progressed on previous BRAF inhibitor monotherapy). Stable disease was noted in ten patients (40%; 95% CI 21-61). Rechallenge with dabrafenib plus trametinib was well tolerated. There were no unexpected or grade 4 or 5 treatment-related adverse events. Grade 3 adverse events occurred in two patients (8%; panniculitis [n=1] and pyrexia [n=1]). Serious adverse events which occurred on study were one patient with an Addison crisis triggered by grade 2 pyrexia symptoms that resolved after discontinuation of dabrafenib and trametinib. No patients died as a result of study treatment. INTERPRETATION: Rechallenge with dabrafenib plus trametinib showed anti-tumour activity in patients who had previously progressed on BRAF inhibitors and as such, rechallenge represents a potential new treatment option for these patients. FUNDING: Vlaamse Liga Tegen Kanker, Novartis.


