LinezolidAntibacterial reagent CAS# 165800-03-3 |
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- NBD-557
Catalog No.:BCC1791
CAS No.:333352-59-3
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 165800-03-3 | SDF | Download SDF |
| PubChem ID | 441401 | Appearance | Powder |
| Formula | C16H20FN3O4 | M.Wt | 337.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PNU 100766, U 100766 | ||
| Solubility | DMSO : ≥ 100 mg/mL (296.43 mM) *"≥" means soluble, but saturation unknown. | ||
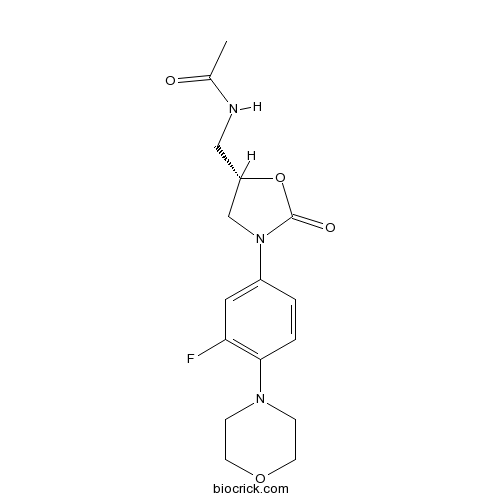
| Chemical Name | N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide | ||
| SMILES | CC(=O)NCC1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F | ||
| Standard InChIKey | TYZROVQLWOKYKF-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C16H20FN3O4/c1-11(21)18-9-13-10-20(16(22)24-13)12-2-3-15(14(17)8-12)19-4-6-23-7-5-19/h2-3,8,13H,4-7,9-10H2,1H3,(H,18,21)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxazolidinone antibiotic. Inhibits bacterial protein synthesis prior to chain initiation. Displays potent antibacterial activity against a variety of multidrug-resistant gram-positive microbes in vitro and in vivo. |

Linezolid Dilution Calculator

Linezolid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9643 mL | 14.8214 mL | 29.6428 mL | 59.2856 mL | 74.107 mL |
| 5 mM | 0.5929 mL | 2.9643 mL | 5.9286 mL | 11.8571 mL | 14.8214 mL |
| 10 mM | 0.2964 mL | 1.4821 mL | 2.9643 mL | 5.9286 mL | 7.4107 mL |
| 50 mM | 0.0593 mL | 0.2964 mL | 0.5929 mL | 1.1857 mL | 1.4821 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2964 mL | 0.5929 mL | 0.7411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxazolidinone antibiotic. Inhibits bacterial protein synthesis prior to chain initiation. Displays potent antibacterial activity against a variety of multidrug-resistant gram-positive microbes in vitro and in vivo.
Licens
- Torososide A
Catalog No.:BCN4694
CAS No.:165689-32-7
- Methyl 6-acetoxyangolensate
Catalog No.:BCN1732
CAS No.:16566-88-4
- L-Stepholidine
Catalog No.:BCN2599
CAS No.:16562-13-3
- 12-O-tetradecanoylphorbol-13-acetate
Catalog No.:BCN2511
CAS No.:16561-29-8
- DEPBT
Catalog No.:BCC2811
CAS No.:165534-43-0
- Di-Dnp-L-Lysine
Catalog No.:BCC8939
CAS No.:1655-49-8
- 16-Hydroxy-2-oxocleroda-3,13-dien-15,16-olide
Catalog No.:BCN1536
CAS No.:165459-53-0
- bis[6-(5,6-dihydrochelerythrinyl)]amine
Catalog No.:BCN8232
CAS No.:165393-48-6
- SA 4503 dihydrochloride
Catalog No.:BCC6339
CAS No.:165377-44-6
- Hemiphroside B
Catalog No.:BCN1731
CAS No.:165338-28-3
- Hemiphroside A
Catalog No.:BCN1730
CAS No.:165338-27-2
- 1-Ethyl-3-nitrophthalate
Catalog No.:BCC8466
CAS No.:16533-45-2
- Eperezolid
Catalog No.:BCC5177
CAS No.:165800-04-4
- 1-Hydroxy-N-methylacridone
Catalog No.:BCN7551
CAS No.:16584-54-6
- Naltrexone
Catalog No.:BCC1783
CAS No.:16590-41-3
- Calystegine A5
Catalog No.:BCN1887
CAS No.:165905-26-0
- (-)-Tetramisole
Catalog No.:BCC4670
CAS No.:16595-80-5
- Z-Thr-OBzl
Catalog No.:BCC2737
CAS No.:16597-50-5
- Cyclogrifolin
Catalog No.:BCN7547
CAS No.:1660156-04-6
- 3,4-O-dimethylcedrusin
Catalog No.:BCN8211
CAS No.:166021-14-3
- Ginkgolic acid C15:0
Catalog No.:BCN2483
CAS No.:16611-84-0
- CCT251545
Catalog No.:BCC6487
CAS No.:1661839-45-7
- Bathophenanthroline
Catalog No.:BCC8840
CAS No.:1662-01-7
- Argentinine
Catalog No.:BCN3987
CAS No.:16625-57-3
Meropenem, levofloxacin and linezolid in human plasma of critical care patients: A fast semi-automated micro-extraction by packed sorbent UHPLC-PDA method for their simultaneous determination.[Pubmed:28371721]
J Pharm Biomed Anal. 2017 Jun 5;140:266-273.
An ultra high-performance liquid chromatographic (UHPLC) method with PDA detection was developed and validated for the simultaneous quantification of meropenem, Linezolid, and levofloxacin in human plasma and applied in human plasma of critical care patients. A semi-automated microextraction by packed sorbent (MEPS) for sample preparation was used. All parameters in the extraction step (pH, sample volume, sample dilution and number of aspiration - ejection cycles) and in the desorption step (percentage of acetonitrile in the solvent of elution and number of aspirations of elution solvent through the device) were statistically significant when the recovery was used as response. The method showed good linearity with correlation coefficients, r(2)>0.9991 for the three drugs, as well as high precision (RSD%<10.83% in each case). Accuracy ranged from -7.8% to +6.7%. The limit of quantification of the three drugs was established at 0.01mug/mL for Linezolid and levofloxacin and 0.02mug/mL for meropenem. Linezolid, meropenem, levofloxacin and the internal standard were extracted from human plasma with a mean recovery ranged from 92.4% to 97.4%. During validation, the concentration of meropenem, Linezolid and levofloxacin was found to be stable after 3 freeze-thaw cycles and for at least 24h after extraction. This method will be subsequently used to quantify the drugs in patients to establish if the dosage regimen given is sufficient to eradicate the infection at the target site.
Predictive score of haematological toxicity in patients treated with linezolid.[Pubmed:28343274]
Eur J Clin Microbiol Infect Dis. 2017 Aug;36(8):1511-1517.
OBJECTIVE: The aims of our study were to determine the factors associated with developing haematological toxicity (HT) in patients taking Linezolid (LZD), to develop a predictive model of HT in these patients, and to evaluate factors associated with 30-day mortality. METHODS: This was an observational retrospective cohort study of patients treated for at least 5 days with LDZ in 2015. Demographic, clinical and analytical data were collected. Development of HT was defined as a 25% platelet count decrease between the basal count and the 1-week lab test. RESULTS: Five hundred forty-nine patients were finally included, mean age was 73.3 (SD 15.4) years, and 303 (55.2%) were men. One hundred seventy-five (30.1%) patients achieved HT criteria during treatment with LZD and 41 (7.5%) died. The final model included the presence of cerebrovascular disease (2 points), moderate or severe liver disease (2 points), renal failure (2 points) and basal platelet count less than 90,000/mm3 (8 points). This new model showed an AUC of 0.711 (IC 95% 0.664-0.757; p < 0.001) to predict the development of HT. The probability of HT based on this classification was 6.2, 29.9 and 76.5% for low (0-4 points), intermediate (5-10 points) and high risk (>10 points), respectively. The independent variables associated with 30-day mortality were metastatic solid tumor, lymphoma, age >75 years and HT. CONCLUSION: This score could help in the identification of patients with high risk for HT and assess the use of an antibiotic other than LZD, an important issue considering its relation with 30-day mortality observed in our study.
Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections.[Pubmed:8576909]
J Med Chem. 1996 Feb 2;39(3):673-9.
Bacterial resistance development has become a very serious clinical problem for many classes of antibiotics. The 3-aryl-2-oxazolidinones are a relatively new class of synthetic antibacterial agents, having a new mechanism of action which involves very early inhibition of bacterial protein synthesis. We have prepared two potent, synthetic oxazolidinones, U-100592 and U-100766, which are currently in clinical development for the treatment of serious multidrug-resistant Gram-positive bacterial infections caused by strains of staphylococci, streptococci, and enterococci. The in vitro and in vivo (po and iv) activities of U-100592 and U-100766 against representative strains are similar to those of vancomycin. U-100592 and U-100766 demonstrate potent in vitro activity against Mycobacterium tuberculosis. A novel and practical asymmetric synthesis of (5S)-(acetamidomethyl)-2-oxazolidinones has been developed and is employed for the synthesis of U-100592 and U-100766. This involves the reaction of N-lithioarylcarbamates with (R)-glycidyl butyrate, resulting in excellent yields and high enantiomeric purity of the intermediate (R)-5-(hydroxymethyl)-2-oxazolidinones.
In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections.[Pubmed:8726028]
Antimicrob Agents Chemother. 1996 Jun;40(6):1508-13.
The Upjohn oxazolidinones, U-100592 and U-100766, are orally bioavailable synthetic antimicrobial agents with spectra of activity against antibiotic-susceptible and -resistant gram-positive pathogens. In several mouse models of methicillin-resistant Staphylococcus aureus infection, U-100592 and U-100766 yielded oral 50% effective doses (ED50) ranging from 1.9 to 8.0 mg/kg of body weight, which compared favorably with vancomycin subcutaneous ED50 values of 1.1 to 4.4 mg/kg. Similarly, both compounds were active versus a Staphylococcus epidermidis experimental systemic infection. U-100592 and U-100766 effectively cured an Enterococcus faecalis systemic infection, with ED50 values of 1.3 and 10.0 mg/kg, and versus a vancomycin-resistant Enterococcus faecium infection in immunocompromised mice, both drugs effected cures at 12.5 and 24.0 mg/kg. Both compounds were exceptionally active in vivo against penicillin- and cephalosporin-resistant Streptococcus pneumoniae, with ED50 values ranging from 1.2 to 11.7 mg/kg in systemic infection models. In soft tissue infection models with S. aureus and E. faecalis, both compounds exhibited acceptable curative activities in the range of 11.0 to 39.0 mg/kg. U-100766 was also very active versus the Bacteroides fragilis soft tissue infection model (ED50 = 46.3 mg/kg). In combination-therapy studies, both U-100592 and U-100766 were indifferent or additive in vivo against a monomicrobic S. aureus infection in combination with other antibiotics active against gram-positive bacteria and combined as readily as vancomycin with gentamicin in the treatment of a polymicrobic S. aureus-Escherichia coli infection. U-100592 and U-100766 are potent oxazolidinones active against antibiotic-susceptible and -resistant gram-positive pathogens in experimental systemic and soft tissue infections.


