Linagliptin (BI-1356)DDP-4 inhibitor,highly potent and competitive CAS# 668270-12-0 |
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- NBD-557
Catalog No.:BCC1791
CAS No.:333352-59-3
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
Number of papers citing our products

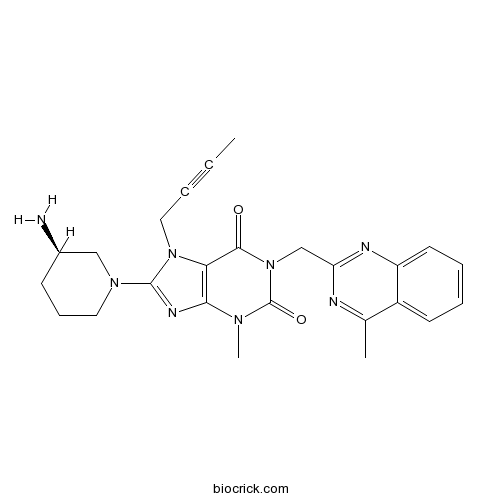
Chemical structure
3D structure
| Cas No. | 668270-12-0 | SDF | Download SDF |
| PubChem ID | 10096344 | Appearance | Powder |
| Formula | C25H28N8O2 | M.Wt | 472.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BI 1356 | ||
| Solubility | DMSO : 10 mg/mL (21.16 mM; Need ultrasonic) | ||
| Chemical Name | 8-[(3R)-3-aminopiperidin-1-yl]-7-but-2-ynyl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]purine-2,6-dione | ||
| SMILES | CC#CCN1C2=C(N=C1N3CCCC(C3)N)N(C(=O)N(C2=O)CC4=NC5=CC=CC=C5C(=N4)C)C | ||
| Standard InChIKey | LTXREWYXXSTFRX-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Linagliptin is a highly potent, competitive inhibitor of dipeptidyl-peptidase 4 (DDP-4) with IC50 value of 1 nM. | |||||
| Targets | DDP-4 | |||||
| IC50 | 1 nM | |||||

Linagliptin (BI-1356) Dilution Calculator

Linagliptin (BI-1356) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1164 mL | 10.582 mL | 21.164 mL | 42.328 mL | 52.9101 mL |
| 5 mM | 0.4233 mL | 2.1164 mL | 4.2328 mL | 8.4656 mL | 10.582 mL |
| 10 mM | 0.2116 mL | 1.0582 mL | 2.1164 mL | 4.2328 mL | 5.291 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4233 mL | 0.8466 mL | 1.0582 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4233 mL | 0.5291 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BI1356 is a potent and competitive DPP-4 inhibitor, which exhibited DPP-4 inhibiting activity in several independent tests with IC50 values of 0.4, 0.5, 0.9, and 1.1nM.[1]
DPP-4 is an N-terminal dipeptidyl exopeptidase existing as a membrane-bound protein and also as a soluble protein in plasma. It plays a major role in the degradation of incretins such as GLP-1 which is of great importance in the process of glucose metabolism. Under physiological conditions, GLP-1 is truncated by DPP-4 rapidly, which is located on the capillary endothelium proximal to the L-cells where GLP-1 is secreted in the ileum. GLP-1 could sensitize β-cells to glucose stimulation,consequently increasing intracellular cAMP concentrations in β-cells and accelerating and augmenting insulin response to absorb glucose. By being measured at the substrate at the binding site, BI1356 inhibits the DPP-4 enzyme.[1]
DPP-4 was extracted from confluent Caco-2 cells to determine the inhibition activity of BI1356. Assays were performed by mixing the inhibitor solution with substrates and the Caco-2 cell extract, which illustrated BI1356 inhibited DPP-4 with IC 50 values of 0.4, 0.5, 0.9, and 1.1 nM. BI1356 also possesses a very significant selectivity for DPP-4 relative to other dipeptidyl peptidases aminopeptidases N and P, prolyloligopeptidase, and the proteases trypsin, plasmin, and thrombin.[1]
An in vivo evaluation showed that BI1356 dose-dependently inhibited the DPP-4 enzyme in plasma within 30 min of administration. Separate doses ranging from 1 to 10 mg/kg achieved significant inhibition activity of DPP-4, which also showed persistent DPP-4 inhibition activity. The ED50 value for inhibition of plasma DPP-4 activity was calculated to be 0.9 mg/kg 24 h post dose. In a clinical study, BI1356 produced a remarkable, clinically meaningful and persistent improvement in glycaemic control, in accordance with enhanced parameters of β-cell function. Patients treated with BI1356 were more likely to achieve a reduction in HbA1c of ≥ 0.5% comparing control.[1,2]
References:
1.Thomas L, Eckhardt M, Langkopf E, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3, 7-dihydro-purine-2, 6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors[J]. Journal of Pharmacology and Experimental Therapeutics, 2008, 325(1): 175-182.
2.Del Prato S, Barnett A H, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of β‐cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial[J]. Diabetes, Obesity and Metabolism, 2011, 13(3): 258-267.
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
- Hernandezine
Catalog No.:BCN7793
CAS No.:6681-13-6
- (+/-)-Forbesione
Catalog No.:BCN6423
CAS No.:667914-50-3
- Syringaresinol-di-O-glucoside
Catalog No.:BCN2600
CAS No.:66791-77-3
- Impurity B of Calcitriol
Catalog No.:BCC1645
CAS No.:66791-71-7
- Platycodin A
Catalog No.:BCN7997
CAS No.:66779-34-8
- Esculin Sesquihydrate
Catalog No.:BCC8324
CAS No.:66778-17-4
- 6,8-Diprenylorobol
Catalog No.:BCN4602
CAS No.:66777-70-6
- Diosbulbin D
Catalog No.:BCN4218
CAS No.:66756-57-8
- NSC 109555 ditosylate
Catalog No.:BCC7540
CAS No.:66748-43-4
- MeBIO
Catalog No.:BCC3898
CAS No.:667463-95-8
- LDN 57444
Catalog No.:BCC2087
CAS No.:668467-91-2
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- H-D-Leu-OBzl.HCl
Catalog No.:BCC2682
CAS No.:66866-69-1
- Talniflumate
Catalog No.:BCC7391
CAS No.:66898-62-2
- 1,2-O-Isopropylidene-beta-D-fructopyranose
Catalog No.:BCN1383
CAS No.:66900-93-4
- α-Conotoxin PIA
Catalog No.:BCC5976
CAS No.:669050-68-4
- Beta-Belladonnine
Catalog No.:BCN1893
CAS No.:6696-63-5
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
- Thiamine hydrochloride
Catalog No.:BCN2225
CAS No.:67-03-8
- EGTA
Catalog No.:BCC7491
CAS No.:67-42-5
- Furazolidone
Catalog No.:BCC8988
CAS No.:67-45-8
- 5-Hydroxymethylfurfural
Catalog No.:BCN4226
CAS No.:67-47-0
Effect of linagliptin (BI 1356) on the steady-state pharmacokinetics of simvastatin.[Pubmed:20497745]
Int J Clin Pharmacol Ther. 2010 Jun;48(6):367-74.
OBJECTIVE: This study was conducted to investigate any potential effect of the dipeptidyl peptidase-4 inhibitor linagliptin (which is being developed to improve glycemic control in patients with Type 2 diabetes) on the pharmacokinetics of simvastatin (a lipid-lowering, HMG-CoA reductase inhibitor). METHODS: This open-label, multiple-dose study was conducted in 20 healthy male Caucasian subjects. Simvastatin (40 mg/day) was administered alone for 6 days, followed by co-administration with linagliptin (10 mg/ day) for 6 days, followed by simvastatin single administration for a further 8 days. Plasma concentrations of simvastatin and its active metabolite simvastatin beta-hydroxy acid were determined on Day 6 (before co-administration of linagliptin) and Days 12, 16 and 20 (after co-administration of linagliptin). RESULTS: The geometric mean ratio (GMR) (90% confidence interval (CI)) following co-administration of linagliptin with simvastatin (Day 12) compared with administration of simvastatin alone (Day 6) for simvastatin AUC was 134.2% (119.4, 150.7) and for simvastatin acid AUC was 133.3% (118.1, 150.3). The GMR (90% CI) for simvastatin Cmax,ss was 110.0% (89.3, 135.6) and for simvastatin acid Cmax,ss was 120.7% (101.5, 143.6). 20 adverse events were reported by 11 subjects. Both simvastatin and linagliptin were well tolerated. CONCLUSIONS: Linagliptin-mediated effects on simvastatin exposure are not considered to be clinically relevant in terms of patient tolerability or safety. Therefore, a dose adjustment of linagliptin is not necessary when these two agents are administered together and linagliptin co-administration is not expected to exert a clinically relevant effect on the pharmacokinetics of other CYP3A4 substrates.
Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes.[Pubmed:21059094]
Diabet Med. 2010 Dec;27(12):1409-19.
AIMS: The efficacy and safety of the dipeptidyl peptidase-4 inhibitor, linagliptin, added to ongoing metformin therapy, were assessed in patients with Type 2 diabetes who had inadequate glycaemic control (HbA(1c) >/= 7.5 to /= 58.5 to
The concentration-dependent binding of linagliptin (BI 1356) and its implication on efficacy and safety.[Pubmed:22541836]
Int J Clin Pharmacol Ther. 2012 May;50(5):323-30.
OBJECTIVES: Linagliptin (BI 1356) is a dipeptidyl peptidase-4 (DPP-4) inhibitor for treatment of Type 2 diabetes which recently gained approval in the US, Europe, and Japan. Linagliptin showed nonlinear pharmacokinetics after intravenous and oral administration, which is due to a concentration-dependent protein binding of linagliptin to its target enzyme DPP-4. The aim of this analysis was to investigate this target-mediated binding of linagliptin and its implication on efficacy and safety. METHODS: Pharmacokinetic modeling and simulations were performed using a two-compartment model with concentration-dependent binding in the central and in one peripheral compartment. The optimum therapeutic dose with minimal off-target side effects was simulated assuming that an antidiabetic effect of linagliptin was due to the linagliptin concentration bound to DPP-4 and that off-target side effects were related to free linagliptin. RESULTS: The difference between steady state AUCs of specifically bound and free linagliptin was maximized at oral doses of 2 - 5 mg. Since plasma DPP-4 inhibition increased slightly from 2.5 to 10 mg, pharmacokinetic simulations and the pharmacodynamic measurements taken together suggest that 5 mg linagliptin could be considered an optimum dose. Simulations with missed doses and additional doses at steady state showed the effect on DPP-4 bound linagliptin and change in DPP-4 inhibition was minimal after missing one 5 mg oral dose of linagliptin while two doses of 5 mg linagliptin resulted in a less than proportional increase of steady state AUC of free linagliptin. CONCLUSIONS: Results from modeling and simulation support a stable antidiabetic effect of linagliptin over 24 h at steady state and further indicate a low risk for off-target side effects.
Pharmacokinetics and pharmacodynamics of single rising intravenous doses (0.5 mg-10 mg) and determination of absolute bioavailability of the dipeptidyl peptidase-4 inhibitor linagliptin (BI 1356) in healthy male subjects.[Pubmed:21053992]
Clin Pharmacokinet. 2010 Dec;49(12):829-40.
BACKGROUND AND OBJECTIVES: Linagliptin (BI 1356) is a highly specific inhibitor of dipeptidyl peptidase (DPP)-4, which is currently in phase III clinical development for the treatment of type 2 diabetes mellitus. Linagliptin exhibits nonlinear pharmacokinetics after oral administration, which are mainly related to concentration-dependent binding of linagliptin to its target, DPP-4. The objectives of the study were to investigate the pharmacokinetics and pharmacodynamics after intravenous administration of linagliptin and to determine its absolute bioavailability (F). SUBJECTS AND METHODS: This was a single rising-dose, randomized, four-group, placebo-controlled, single-blind (within dose groups) study. Thirty-six healthy men aged 18-50 years were enrolled and randomized into four sequential treatment groups. Group 1 received linagliptin 0.5 mg intravenously, group 2 received 2.5 mg intravenously and group 4 received 10 mg intravenously. In group 3, subjects underwent a two-way randomized crossover, receiving 5 mg intravenously and a 10 mg oral tablet. Linagliptin concentrations in plasma and urine, as well as plasma DPP-4 activity, were determined by validated assays. Noncompartmental analysis and population pharmacokinetic modelling were performed. RESULTS: Linagliptin showed nonlinear pharmacokinetics after intravenous infusion of 0.5-10 mg, with a less than dose-proportional increase in exposure. Noncompartmental parameters were calculated on the basis of total (i.e. bound and unbound) plasma concentrations. The total clearance value was low and increased with dose from 2.51 to 14.3 L/h. The apparent steady-state volume of distribution (V(ss)) increased with dose from 380 to 1540 L. Renal excretion of the unchanged parent compound increased with increasing plasma concentrations from 2.72% in the 0.5 mg dose group to 23.0% in the 10 mg dose group. The terminal elimination half-life was comparable across dose groups (126-139 hours). Because of the nonlinear pharmacokinetics, the standard approach of comparing the area under the plasma concentration-time curve (AUC) after oral administration with the AUC after intravenous administration led to dose-dependent estimates of the absolute bioavailability. Therefore, a population pharmacokinetic model was developed, accounting for the concentration-dependent protein binding of linagliptin to its target enzyme, DPP-4. The model-derived estimates of the V(ss) and clearance of linagliptin not bound to DPP-4 were 402.2 L and 26.9 L/h, respectively. The absolute bioavailability was estimated to be about 30% for the linagliptin 10 mg tablet. CONCLUSION: The nonlinear pharmacokinetic characteristics and the pharmacokinetic/pharmacodynamic relationship of linagliptin were independent of the mode of administration (intravenous or oral). Because of the nonlinear pharmacokinetics, the standard approach of comparing the AUC after oral administration with the AUC after intravenous administration was inappropriate to determine the absolute bioavailability of linagliptin. By a modelling approach, the absolute bioavailability of the 10 mg linagliptin tablet was estimated to be about 30%.


