Lavendustin AEGFR, p60c-src inhibitor CAS# 125697-92-9 |
- CUDC-101
Catalog No.:BCC2149
CAS No.:1012054-59-9
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 125697-92-9 | SDF | Download SDF |
| PubChem ID | 3894 | Appearance | Powder |
| Formula | C21H19NO6 | M.Wt | 381.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | RG 14355 | ||
| Solubility | DMSO : ≥ 250 mg/mL (655.51 mM) *"≥" means soluble, but saturation unknown. | ||
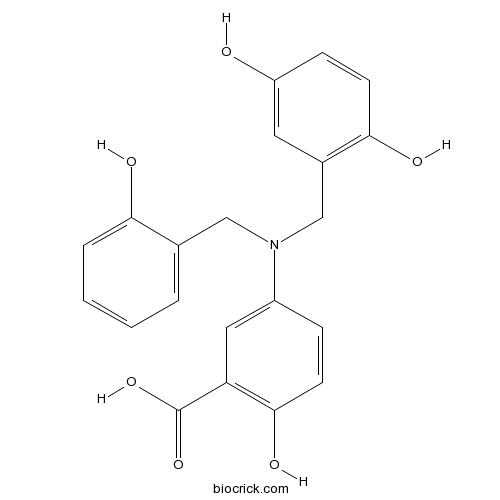
| Chemical Name | 5-[(2,5-dihydroxyphenyl)methyl-[(2-hydroxyphenyl)methyl]amino]-2-hydroxybenzoic acid | ||
| SMILES | C1=CC=C(C(=C1)CN(CC2=C(C=CC(=C2)O)O)C3=CC(=C(C=C3)O)C(=O)O)O | ||
| Standard InChIKey | ULTTYPMRMMDONC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19NO6/c23-16-6-8-19(25)14(9-16)12-22(11-13-3-1-2-4-18(13)24)15-5-7-20(26)17(10-15)21(27)28/h1-10,23-26H,11-12H2,(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lavendustin A and hormothamnione exhibit cytotoxic effects on tumor cell lines. 2. Lavendustin A is a potent tyrosine kinase inhibitor of the epidermal growth factor (EGF) receptor, and an angiogenesis inhibitor. 3. Lavendustin A is a hyperbolic mixed-type inhibitor with respect to both ATP and the peptide substrate, with a major effect on the binding affinities for both substrates. 4. Lavendustin A at 0.1 nM-1 microM causes a concave-shaped inhibition of the insulin release stimulated by 7 mM glucose, the inhibitory effect can be overcomed by higher concentrations of glucose. |
| Targets | EGFR | PKC | PI3K | Calcium Channel |

Lavendustin A Dilution Calculator

Lavendustin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6221 mL | 13.1103 mL | 26.2206 mL | 52.4411 mL | 65.5514 mL |
| 5 mM | 0.5244 mL | 2.6221 mL | 5.2441 mL | 10.4882 mL | 13.1103 mL |
| 10 mM | 0.2622 mL | 1.311 mL | 2.6221 mL | 5.2441 mL | 6.5551 mL |
| 50 mM | 0.0524 mL | 0.2622 mL | 0.5244 mL | 1.0488 mL | 1.311 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2622 mL | 0.5244 mL | 0.6555 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lavendustin B
Catalog No.:BCN1809
CAS No.:125697-91-8
- CDK4 inhibitor
Catalog No.:BCC4242
CAS No.:1256963-02-6
- 2''-O-acetyl-platyconic acid A
Catalog No.:BCN3318
CAS No.:1256935-30-4
- 3''-O-acetyl-platyconic acid A
Catalog No.:BCN3319
CAS No.:1256935-28-0
- Blinin
Catalog No.:BCN8455
CAS No.:125675-09-4
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- Ledipasvir
Catalog No.:BCC1696
CAS No.:1256388-51-8
- RuBi-Nicotine
Catalog No.:BCC7793
CAS No.:1256362-30-7
- AI-10-49
Catalog No.:BCC3973
CAS No.:1256094-72-0
- TRX818
Catalog No.:BCC6458
CAS No.:1256037-58-7
- [Leu31,Pro34]-Neuropeptide Y (porcine)
Catalog No.:BCC5716
CAS No.:125580-28-1
- Rotigotine hydrochloride
Catalog No.:BCC1908
CAS No.:125572-93-2
- (-)-Epicatechin gallate
Catalog No.:BCN6327
CAS No.:1257-08-5
- TBTU
Catalog No.:BCC2823
CAS No.:125700-67-6
- TDBTU
Catalog No.:BCC2825
CAS No.:125700-69-8
- TPTU
Catalog No.:BCC2827
CAS No.:125700-71-2
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- TC-SP 14
Catalog No.:BCC7926
CAS No.:1257093-40-5
- TC-G 24
Catalog No.:BCC6146
CAS No.:1257256-44-2
- NPEC-caged-noradrenalin
Catalog No.:BCC7835
CAS No.:1257323-83-3
- NPEC-caged-(S)-AMPA
Catalog No.:BCC7789
CAS No.:1257323-84-4
- NPEC-caged-(S)-3,4-DCPG
Catalog No.:BCC7652
CAS No.:1257323-85-5
- NPEC-caged-serotonin
Catalog No.:BCC7836
CAS No.:1257326-22-9
- NPEC-caged-dopamine
Catalog No.:BCC7837
CAS No.:1257326-23-0
Synthesis, anticancer activity, and inhibition of tubulin polymerization by conformationally restricted analogues of lavendustin A.[Pubmed:12699385]
J Med Chem. 2003 Apr 24;46(9):1670-82.
Compounds in the Lavendustin A series have been shown to inhibit both protein-tyrosine kinases (PTKs) and tubulin polymerization. Since certain Lavendustin A derivatives can exist in conformations that resemble both the trans-stilbene structure of the PTK inhibitor piceatannol and the cis-stilbene structure of the tubulin polymerization inhibitor combretastatin A-4, the possibility exists that the ratio of the two types of activities of the lavendustins could be influenced through the synthesis of conformationally restricted analogues. Accordingly, the benzylaniline structure of a series of pharmacologically active Lavendustin A fragments was replaced by either their cis- or their trans-stilbene relatives, and effects on both inhibition of tubulin polymerization and cytotoxicity in cancer cell cultures were monitored. Both dihydrostilbene and 1,2-diphenylalkyne congeners were also prepared and evaluated biologically. Surprisingly, conformational restriction of the bridge between the two aromatic rings of the lavendustins had no significant effect on biological activity. On the other hand, conversion of the three phenolic hydroxyl groups of the Lavendustin A derivatives to their corresponding methyl ethers consistently abolished their ability to inhibit tubulin polymerization and usually decreased cytotoxicity in cancer cell cultures as well, indicating the importance of at least one of the phenolic hydroxyl groups. Further investigation suggested that the phenolic hydroxyl group in the salicylamide ring was required for activity, while the two phenol moieties in the hydroquinone ring could be methylated with retention of activity. Two of the Lavendustin A derivatives displayed IC(50) values of 1.4 microM for inhibition of tubulin polymerization, which ranks them among the most potent of the known tubulin polymerization inhibitors.
Modulation of tyrosine kinase activity has multiple actions on insulin release from the pancreatic beta-cell: studies with lavendustin A.[Pubmed:9243329]
Jpn J Pharmacol. 1997 Jun;74(2):203-8.
We investigated the role of tyrosine kinases in the regulation of insulin release from a hamster beta-cell line, HIT T15, using selective tyrosine kinase inhibitors. Genistein increased the insulin release induced by glucose, but herbimycin A, tyrphostins and the erbstatin analogue failed to change the release. Lavendustin A at 0.1 nM-1 microM caused a concave-shaped inhibition of the insulin release stimulated by 7 mM glucose. The inhibitory effect of Lavendustin A was overcome by higher concentrations of glucose. Lavendustin B, the negative control analogue, had no effect on the release. Lavendustin A at a nanomolar range progressively inhibited insulin release by high K+ (50 mM)-depolarization, whereas the inhibitor did not change the insulin release by Ca2+ ionophore (A23187). On the contrary, Lavendustin A at 10 nM significantly increased insulin release when glucose-induced insulin release was enhanced by either 5 microM forskolin or 162 nM 12-O-tetradecanoylphorbol 13-acetate. Lavendustin A failed to influence the Ca(2+)-induced insulin release from HIT cells permeabilized with streptolysin-O. These findings suggest that tyrosine kinases may play versatile roles in the control of insulin release from the pancreatic beta-cell.
Kinetic analysis of the inhibition of the epidermal growth factor receptor tyrosine kinase by Lavendustin-A and its analogue.[Pubmed:1939153]
J Biol Chem. 1991 Nov 5;266(31):21105-12.
Lavendustin-A was reported to be a potent tyrosine kinase inhibitor of the epidermal growth factor (EGF) receptor (Onoda, T., Iinuma, H., Sasaki, Y., Hamada, M., Isshibi, K., Naganawa, H., Takeuchi, T., Tatsuta, K., and Umezawa, K. (1989) J. Nat. Prod. 52, 1252-1257). Its inhibition kinetics was studied in detail using the baculovirus-expressed recombinant intracellular domain of the EGF receptor (EGFR-IC). Lavendustin-A (RG 14355) is a slow and tight binding inhibitor of the receptor tyrosine kinase. The pre-steady state kinetic analysis demonstrates that the inhibition corresponds to a two-step mechanism in which an initial enzyme-inhibitor complex (EI) is rapidly formed followed by a slow isomerization step to form a tight complex (EI*). The dissociation constant for the initial rapid forming complex is 370 nM, whereas the overall dissociation constant is estimated to be less than or equal to 1 nM. The difference between the two values is due to the tight binding nature of the inhibitor to the enzyme in EI*. The kinetic analysis using a preincubation protocol to pre-equilibrate the enzyme with the inhibitor in the presence of one substrate showed that Lavendustin-A is a hyperbolic mixed-type inhibitor with respect to both ATP and the peptide substrate, with a major effect on the binding affinities for both substrates. An analogue of Lavendustin-A (RG 14467) showed similar inhibition kinetics to that of Lavendustin-A. The results of the pre-steady state analysis are also consistent with the proposed two-step mechanism. The dissociation constant for the initial fast forming complex in this case is 3.4 microM, whereas the overall dissociation constant is estimated to be less than or equal to 30 nM. It is a partial (hyperbolic) competitive inhibitor with respect to ATP. Its inhibition is reduced to different extents by different peptide substrates, when the peptide is added to the enzyme simultaneously with the inhibitor. When studied with the least protective peptide, K1 (a peptide containing the major autophosphorylation site of the EGF receptor), RG 14467 acts as a hyperbolic noncompetitive inhibitor with respect to the peptide.
Angiotensin II stimulation of Ca2+-channel current in vascular smooth muscle cells is inhibited by lavendustin-A and LY-294002.[Pubmed:9914387]
Pflugers Arch. 1999 Feb;437(3):317-23.
Angiotensin II (AngII) is coupled to several important intracellular signaling pathways, and increases intracellular Ca2+. In vascular smooth muscle (VSM) cells, AngII is known to activate enzymes such as tyrosine protein kinase (Tyr-PK), phospholipase C (PLC), protein kinase C (PKC), and phophatidylinositol-3-kinase (PI-3-K). A non-receptor Tyr-PK, pp60(c-src), and PKC have been reported to stimulate the Ca2+ channels in VSM cells. However, less is known about AngII action on the voltage-gated Ca2+ channels. The Ca2+-channel currents of a cultured rat aortic smooth muscle cell line, A7r5, were recorded using whole-cell voltage clamp. Application of 50 nM AngII significantly increased the amplitude of Ba2+ currents through the voltage-gated Ca2+ channels (IBa) by 34. 5+/-9.1% (n=10) within 1 min. In the presence of lavendustin-A (5 microM), a selective inhibitor of Tyr-PK, AngII failed to stimulate IBa (n=5). AngII stimulation of IBa was also prevented by (5 microM) LY-294002, an inhibitor of PI-3-K (n=5). In contrast, H-7 (30 microM), an inhibitor of PKC, did not prevent the effect of AngII on IBa (n=6). These results suggest that AngII may stimulate the Ca2+ channels of VSM cells through Tyr-PK and PI-3-K under conditions that probably exclude participation of PK-C.
Synthesis and anticancer activity of chromone-based analogs of lavendustin A.[Pubmed:20630626]
Eur J Med Chem. 2010 Sep;45(9):4288-92.
Lavendustin A and hormothamnione were reported to exhibit cytotoxic effects on tumor cell lines. In the present studies, a series of chromone-based Lavendustin Analogs were synthesized as a simplified hybrid of hormothamnione and Lavendustin A by the reductive-amination of formyl-chromone 5 with various amines followed by aminoalkylation. Most compounds synthesized showed significantly improved potencies compared to the standard compound 3 against most of cancer cell lines tested indicating that the removal of styryl group enhanced cancer cell growth inhibitory activities. Compound 4h and 4k showed the most potent inhibitory activities with GI(50) values in the range of 6.01-9.92 microg/ml on A-549 and HCT-15 cells.
Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors.[Pubmed:1656271]
Nature. 1991 Oct 10;353(6344):558-60.
Long-term potentiation (LTP) in the hippocampus is thought to contribute to memory formation. In the Ca1 region, LTP requires the NMDA (N-methyl-D-aspartate) receptor-dependent influx of Ca2+ and activation of serine and threonine protein kinases. Because of the high amount of protein tyrosine kinases in hippocampus and cerebellum, two regions implicated in learning and memory, we examined the possible additional requirement of tyrosine kinase activity in LTP. We first examined the specificity in brain of five inhibitors of tyrosine kinase and found that two of them, Lavendustin A and genistein, showed substantially greater specificity for tyrosine kinase from hippocampus than for three serine-threonine kinases: protein kinase A, protein kinase C, and Ca2+/calmodulin kinase II. Lavendustin A and genistein selectively blocked the induction of LTP when applied in the bath or injected into the postsynaptic cell. By contrast, the inhibitors had no effect on the established LTP, on normal synaptic transmission, or on the neurotransmitter actions attributable to the actions of protein kinase A or protein kinase C. These data suggest that tyrosine kinase activity could be required postsynaptically for long-term synaptic plasticity in the hippocampus. As Ca2+ calmodulin kinase II or protein kinase C seem also to be required, the tyrosine kinases could participate postsynaptically in a kinase network together with serine and threonine kinases.
Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus.[Pubmed:2614420]
J Nat Prod. 1989 Nov-Dec;52(6):1252-7.
A potent tyrosine kinase inhibitor, Lavendustin A [1], has been isolated from a butyl acetate extract of Streptomyces griseolavendus culture filtrate. It inhibits epidermal growth factor receptor-associated tyrosine kinase with an IC50 of 4.4 ng/ml, which is about 50 times more inhibitory than erbstatin. It does not inhibit protein kinase A or C. Its structure, determined by spectral data and total synthesis, is novel, having a tertiary amine in the center with substituted benzyl and phenyl groups. Lavendustin A competes with ATP and is noncompetitive with the peptide. Its structure-activity relationship is discussed.


