LY2183240Blocker of anandamide uptake,highly potent CAS# 874902-19-9 |
- PF-04457845
Catalog No.:BCC1851
CAS No.:1020315-31-4
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- FAAH inhibitor 1
Catalog No.:BCC4254
CAS No.:326866-17-5
- URB597
Catalog No.:BCC2324
CAS No.:546141-08-6
- JNJ-1661010
Catalog No.:BCC2315
CAS No.:681136-29-8
Quality Control & MSDS
Number of papers citing our products

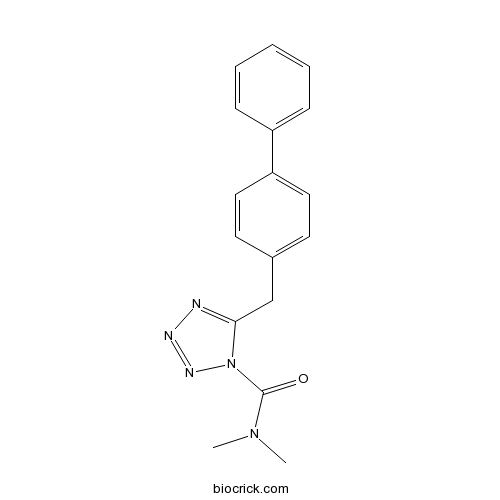
Chemical structure
3D structure
| Cas No. | 874902-19-9 | SDF | Download SDF |
| PubChem ID | 11507802 | Appearance | Powder |
| Formula | C17H17N5O | M.Wt | 307.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (162.68 mM; Need ultrasonic) | ||
| Chemical Name | N,N-dimethyl-5-[(4-phenylphenyl)methyl]tetrazole-1-carboxamide | ||
| SMILES | CN(C)C(=O)N1C(=NN=N1)CC2=CC=C(C=C2)C3=CC=CC=C3 | ||
| Standard InChIKey | GZNIYOXWFCDBBJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H17N5O/c1-21(2)17(23)22-16(18-19-20-22)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11H,12H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY2183240 is a novel and highly potent blocker of anandamide uptake (IC50 = 270 pM). LY2183240 inhibits fatty acid amide hydrolase (FAAH) activity (IC50 = 12.4 nM).
IC50: 270 pM (anandamide uptake); 12.4 nM (FAAH)
Target: FAAH; Anandamide uptake
Following i.p. administration in rats, LY2183240 increases brain anandamide concentration and exerts antinociceptive effects in formalin model of pain. References: | |||||

LY2183240 Dilution Calculator

LY2183240 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2536 mL | 16.2681 mL | 32.5362 mL | 65.0724 mL | 81.3405 mL |
| 5 mM | 0.6507 mL | 3.2536 mL | 6.5072 mL | 13.0145 mL | 16.2681 mL |
| 10 mM | 0.3254 mL | 1.6268 mL | 3.2536 mL | 6.5072 mL | 8.134 mL |
| 50 mM | 0.0651 mL | 0.3254 mL | 0.6507 mL | 1.3014 mL | 1.6268 mL |
| 100 mM | 0.0325 mL | 0.1627 mL | 0.3254 mL | 0.6507 mL | 0.8134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2183240 is a highly potent blocker of anandamide uptake with IC50 value of 270 pM, and an inhibitor of fatty acid amide hydrolase (FAAH) activity with IC50 value of 12.4 nM [1].
Fatty acid amide hydrolase (FAAH) belongs to the family of serine hydrolase, which is an integral membrane enzyme. It has esterase and amidase activity that are responsive for the uptake of fatty acid amide (FAA) family signaling lipids, including anandamide. The uptake and metabolic process of anandamide is mainly achieved by FAAH, therefore the inhibition of FAAH may result in the blockage of anadaminde uptake.
LY2183240 is a potent and covalent inhibitor of FAAH, with the effect of blocking anandamide uptake. Biochemical research had identified that LY2183240 inactivate FAAH via carbamylation of the serine nucleophile of FAAH [2]. In RBL cell line containing FAAH, radioactive labeled 3H-LY2183240 exhibited strong binding to FAAH, inhibition of FAAH and thus blockage of anandamide uptake. However, in HeLa cells lacking FAAH, 3H-LY2183240 was less bound, and the blockage of anandamide uptake was very weak. It suggested that the direct interaction between LY2183240 and FAAH was required to block the anandamide uptake. However, it also indicated that there might be uncharacterized pathways of anandamide uptake regulated by LY2183240, independent from FAAH [1]. However, FAAH is not the sole target for LY2183240. Several uncharacterized brain serine hydrolases were also identified as the target of LY2183240, and these might be additional pathway for anandamide uptake [2].
In mouse model, 10 mg/kg injection of LY2183240 would produce analgesic effects in the formalin test of noxious pain which is a phenotype associated with elevated level of anandamide in brain. It indicated LY2183240 might inhibit FAAH in vivo [2]. When mice were administrated with LY2183240 (10 mg/kg, ip) for 90 min, characterization of brain tissues revealed that LY2183240 inactivated FAAH and other brain serine hydrolases ranging from 25-35 kDa [2].
References:
[1] Dickason-Chesterfield A K et al. , Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors. Cell Mol Neurobiol. 2006, 26(4-6): 407-23.
[2] Alexander J P, Cravatt B F. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc. 2006, 128(30): 9699-9704.
- L-741,742 hydrochloride
Catalog No.:BCC5696
CAS No.:874882-93-6
- Isamoltane hemifumarate
Catalog No.:BCC6879
CAS No.:874882-92-5
- Toceranib phosphate
Catalog No.:BCC2006
CAS No.:874819-74-6
- Dihydroajugapitin
Catalog No.:BCN4421
CAS No.:87480-84-0
- Liriope muscari baily saponins C
Catalog No.:BCN2340
CAS No.:87480-46-4
- H-Arg-OtBu.2HCl
Catalog No.:BCC2862
CAS No.:87459-72-1
- (25RS)-Ruscogenin
Catalog No.:BCN7805
CAS No.:874485-32-2
- Schizanrin L
Catalog No.:BCN3620
CAS No.:874472-16-9
- SEN 12333
Catalog No.:BCC6182
CAS No.:874450-44-9
- 11(13)-Dehydroivaxillin
Catalog No.:BCN4420
CAS No.:87441-73-4
- Viteralone
Catalog No.:BCN4419
CAS No.:87440-75-3
- 6-Dehydroxy-8-hydroxygaleopsinolone
Catalog No.:BCN7402
CAS No.:87440-66-2
- ZK 756326
Catalog No.:BCC7413
CAS No.:874911-96-3
- Boc-D-Cys(Trt)-OH
Catalog No.:BCC3381
CAS No.:87494-13-1
- H-D-Phg-OH
Catalog No.:BCC3313
CAS No.:875-74-1
- Tenacissoside X
Catalog No.:BCN8354
CAS No.:875057-87-7
- YIL 781
Catalog No.:BCC7826
CAS No.:875258-85-8
- Lusianthridin
Catalog No.:BCN3689
CAS No.:87530-30-1
- Dimethyl lithospermate B
Catalog No.:BCN2823
CAS No.:875313-64-7
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- MGCD-265
Catalog No.:BCC2479
CAS No.:875337-44-3
- Anacetrapib (MK-0859)
Catalog No.:BCC2327
CAS No.:875446-37-0
- H-D-Tyr-OtBu
Catalog No.:BCC3136
CAS No.:87553-74-0
- ent-14,15-Dinor-13-oxolabda-8(17),11-dien-18-oic acid
Catalog No.:BCN1319
CAS No.:875585-30-1
The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases.[Pubmed:16866524]
J Am Chem Soc. 2006 Aug 2;128(30):9699-704.
How lipid transmitters move within and between cells to communicate signals remains an important and largely unanswered question. Integral membrane transporters, soluble lipid-binding proteins, and metabolic enzymes have all been proposed to collaboratively regulate lipid signaling dynamics in vivo. Assignment of the relative contributions made by each of these classes of proteins requires selective pharmacological agents to perturb their individual functions. Recently, LY2183240, a heterocyclic urea inhibitor of the putative endocannabinoid (EC) transporter, was shown to disrupt the cellular uptake of the lipid EC anandamide and promote analgesia in vivo. Here, we show that LY2183240 is a potent, covalent inhibitor of the EC-degrading enzyme fatty acid amide hydrolase (FAAH). LY2183240 inactivates FAAH by carbamylation of the enzyme's serine nucleophile. More global screens using activity-based proteomic probes identified several additional serine hydrolases that are also inhibited by LY2183240. These results indicate that the blockade of anandamide uptake observed with LY2183240 may be due primarily to the inactivation of FAAH, providing further evidence that this enzyme serves as a metabolic driving force that promotes the diffusion of anandamide into cells. More generally, the proteome-wide target promiscuity of LY2183240 designates the heterocyclic urea as a chemotype with potentially excessive protein reactivity for drug design.
Effects of the novel endocannabinoid uptake inhibitor, LY2183240, on fear-potentiated startle and alcohol-seeking behaviors in mice selectively bred for high alcohol preference.[Pubmed:20838777]
Psychopharmacology (Berl). 2010 Dec;212(4):571-83.
RATIONALE: Alcohol-use disorders often occur together with anxiety disorders in humans which may be partly due to common inherited genetic factors. Evidence suggests that the endocannabinoid system (ECS) is a promising therapeutic target for the treatment of individuals with anxiety and/or alcohol-use disorders. OBJECTIVES: The present study assessed the effects of a novel endocannabinoid uptake inhibitor, LY2183240, on anxiety- and alcohol-seeking behaviors in a unique animal model that may represent increased genetic risk to develop co-morbid anxiety and alcohol-use disorders in humans. Mice selectively bred for high alcohol preference (HAP) show greater fear-potentiated startle (FPS) than mice selectively bred for low alcohol preference (LAP). We examined the effects of LY2183240 on the expression of FPS in HAP and LAP mice and on alcohol-induced conditioned place preference (CPP) and limited-access alcohol drinking behavior in HAP mice. RESULTS: Repeated administration of LY2183240 (30 mg/kg) reduced the expression of FPS in HAP but not LAP mice when given prior to a second FPS test 48 h after fear conditioning. Both the 10 and 30 mg/kg doses of LY2183240 enhanced the expression of alcohol-induced CPP and this effect persisted in the absence of the drug. LY2183240 did not alter limited-access alcohol drinking behavior, unconditioned startle responding, or locomotor activity. CONCLUSIONS: These findings suggest that ECS modulation influences both conditioned fear and conditioned alcohol reward behavior. LY2183240 may be an effective pharmacotherapy for individuals with anxiety disorders, such as post-traumatic stress disorder, but may not be appropriate for individuals with co-morbid anxiety and alcohol-use disorders.
Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors.[Pubmed:16736384]
Cell Mol Neurobiol. 2006 Jul-Aug;26(4-6):407-23.
: 1. The mechanism of anandamide uptake and disposal has been an issue of considerable debate in the cannabinoid field. Several compounds have been reported to inhibit anandamide uptake or fatty acid amide hydrolase (FAAH; the primary catabolic enzyme of anandamide) activity with varying degrees of potency and selectivity. We recently reported the first evidence of a binding site involved in the uptake of endocannabinoids that is independent from FAAH. There are no direct comparisons of purported selective inhibitory compounds in common assay conditions measuring anandamide uptake, FAAH activity and binding activity. 2. A subset of compounds reported in the literature were tested in our laboratory under common assay conditions to measure their ability to (a) inhibit [(14)C]-anandamide uptake in cells containing (RBL-2H3) or cells lacking (HeLa) FAAH, (b) inhibit purified FAAH hydrolytic activity, and (c) inhibit binding to a putative binding site involved in endocannabinoid transport in both RBL and HeLa cell membranes. 3. Under these conditions, nearly all compounds tested inhibited (a) uptake of [(14)C]-anandamide, (b) enzyme activity in purified FAAH preparations, and (c) radioligand binding of [(3)H]-LY2183240 in RBL and HeLa plasma membrane preparations. General rank order potency was preserved within the three assays. However, concentration response curves were right-shifted for functional [(14)C]-anandamide uptake in HeLa (FAAH(-/-)) cells. 4. A more direct comparison of multiple inhibitors could be made in these three assay systems performed in the same laboratory, revealing more information about the selectivity of these compounds and the relationship between the putative endocannabinoid transport protein and FAAH. At least two separate proteins appear to be involved in uptake and degradation of anandamide. The most potent inhibitory compounds were right-shifted when transport was measured in HeLa (FAAH(-/-)) cells suggesting a requirement for a direct interaction with the FAAH protein to maintain high affinity binding of anandamide or inhibitors to the putative anandamide transport protein.


