L-trans-2,4-PDCTransportable EAAT1-4 inhibitor/non-transportable EAAT5 inhibitor CAS# 64769-66-0 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 64769-66-0 | SDF | Download SDF |
| PubChem ID | 1515192 | Appearance | Powder |
| Formula | C6H9NO4 | M.Wt | 159.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | <em>trans</em>-4-Carboxy-L-proline | ||
| Solubility | Soluble to 100 mM in water | ||
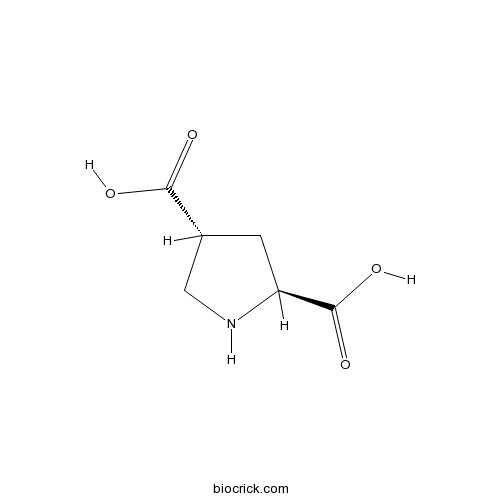
| Chemical Name | (2S,4R)-pyrrolidine-2,4-dicarboxylic acid | ||
| SMILES | C1C(CNC1C(=O)O)C(=O)O | ||
| Standard InChIKey | NRSBQSJHFYZIPH-DMTCNVIQSA-N | ||
| Standard InChI | InChI=1S/C6H9NO4/c8-5(9)3-1-4(6(10)11)7-2-3/h3-4,7H,1-2H2,(H,8,9)(H,10,11)/t3-,4+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, competitive, transportable EAAT1-4 inhibitor/non-transportable EAAT5 inhibitor. Also available as part of the Excitatory Amino Acid Transporter Inhibitor. |

L-trans-2,4-PDC Dilution Calculator

L-trans-2,4-PDC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.2838 mL | 31.4189 mL | 62.8378 mL | 125.6755 mL | 157.0944 mL |
| 5 mM | 1.2568 mL | 6.2838 mL | 12.5676 mL | 25.1351 mL | 31.4189 mL |
| 10 mM | 0.6284 mL | 3.1419 mL | 6.2838 mL | 12.5676 mL | 15.7094 mL |
| 50 mM | 0.1257 mL | 0.6284 mL | 1.2568 mL | 2.5135 mL | 3.1419 mL |
| 100 mM | 0.0628 mL | 0.3142 mL | 0.6284 mL | 1.2568 mL | 1.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Hydroxy-1,2,3,7-tetramethoxyxanthone
Catalog No.:BCN7565
CAS No.:64756-87-2
- 1,2,3,6,7-Pentamethoxyxanthone
Catalog No.:BCN7525
CAS No.:64756-86-1
- Tetrahydroalstonine
Catalog No.:BCN4189
CAS No.:6474-90-4
- RA-V
Catalog No.:BCN3513
CAS No.:64725-24-2
- Morin dihydrate
Catalog No.:BCN8156
CAS No.:6472-38-4
- Cinaciguat hydrochloride
Catalog No.:BCC8096
CAS No.:646995-35-9
- Dihydroflavokawain C
Catalog No.:BCC9229
CAS No.:
- Demethylwedelolactone
Catalog No.:BCN2663
CAS No.:6468-55-9
- Coleonol B
Catalog No.:BCN4188
CAS No.:64657-21-2
- 1,9-Dideoxyforskolin
Catalog No.:BCC6352
CAS No.:64657-18-7
- Demethylluvangetin
Catalog No.:BCN7569
CAS No.:64652-10-4
- VcMMAE
Catalog No.:BCC2033
CAS No.:646502-53-6
- Betulalbuside A
Catalog No.:BCN4190
CAS No.:64776-96-1
- Trilobatin 2''-acetate
Catalog No.:BCN4191
CAS No.:647853-82-5
- Glucagon (19-29), human
Catalog No.:BCC1012
CAS No.:64790-15-4
- Heraclenol acetonide
Catalog No.:BCN4192
CAS No.:64790-68-7
- Neomangiferin
Catalog No.:BCN4970
CAS No.:64809-67-2
- Vitexin-2''-O-rhamnoside
Catalog No.:BCN5025
CAS No.:64820-99-1
- ophocarpine hydrobromide
Catalog No.:BCN7541
CAS No.:78003-71-1
- AS-604850
Catalog No.:BCC4989
CAS No.:648449-76-7
- AS-605240
Catalog No.:BCC2495
CAS No.:648450-29-7
- Rubusoside
Catalog No.:BCN2313
CAS No.:64849-39-4
- Urapidil HCl
Catalog No.:BCC5044
CAS No.:64887-14-5
- trans-Norpterosin C
Catalog No.:BCN6918
CAS No.:64890-70-6
Activity dependent internalization of the glutamate transporter GLT-1 mediated by beta-arrestin 1 and ubiquitination.[Pubmed:27044663]
Neuropharmacology. 2016 Aug;107:376-386.
GLT-1 is the main glutamate transporter in the brain and undergoes trafficking processes that control its concentration on the cell surface thereby shaping glutamatergic neurotransmission. We have investigated how the traffic of GLT-1 is regulated by transporter activity. We report that internalization of GLT-1 from the cell surface is accelerated by transportable substrates like glutamate or aspartate, as well as by the transportable inhibitor L-trans-2,4-PDC, but not by the non-substrate inhibitor WAY 213613 in primary mixed cultures and in transiently transfected HEK293 cells. Analysis of the mechanism of endocytosis in HEK293 cells revealed that glutamate promoted the association with the transporter of the adaptor protein beta-arrestin and the ubiquitin ligase Nedd4-2. The addition of glutamate is accompanied by an increase in the transporter ubiquitination, and the internalization is suppressed by an ubiquitination inhibitor (PYR41), and in a mutant defective in C-terminal lysines. The glutamate triggered endocytosis was also suppressed by siRNA for beta-arrestin. This regulatory mechanism might be relevant in controlling the amount of transporter on the cell surface in conditions such as ischemia or traumatic brain injury, where extracellular concentrations of glutamate are persistently elevated.
Effects of theanine, r-glutamylethylamide, on neurotransmitter release and its relationship with glutamic acid neurotransmission.[Pubmed:16493792]
Nutr Neurosci. 2005 Aug;8(4):219-26.
t Theanine, r-glutamylethylamide, is one of the major amino acid components in green tea and many researchers have compared theanine's effects with glutamic acid because the chemical structure is similar. In the previous study, we demonstrated that theanine can pass brain-blood barrier and may play as an agonist or an antagonist of some receptors. In this study, we investigated the effects of theanine on neurotransmitter release in the rat brain striatum by in vivo brain microdialysis and examined whether theanine affected glutamate transporters by comparing it with a glutamate transporter blocker, L-trans-Pyrrolidine-2,4-dicarboxylic acid (L-trans-2,4-PDC). Because we investigated whether the effects of theanine is similar to L-trans-2,4-PDC on the brain neurotransmission, we measured dopamine release and some amino acids release which are known as excitatory or inhibitory neurotransmitters from neurons by theanine or L-trans-2,4-PDC perfusion into the rat brain striatum. L-trans-2,4-PDC or theanine perfusion into the brain striatum caused dopamine release from dopaminergic neurons. In addition, L-trans-2,4-PDC perfusion increased glutamic acid, aspartic acid and, whereas theanine perfusion prevented aspartic acid release and increased glycine release. These results suggested that the mechanism of dopamine release caused by theanine is different from glutamate transporter blockers or glutamic acid. Further, L-trans-2,4-PDC cause excitatory neurotransmission, whereas theanine may inhibit excitatory neurotransmission and cause inhibitory neurotransmission via glycine receptors.
Effects of NMDA receptor antagonists on olfactory learning and memory in the honeybee (Apis mellifera).[Pubmed:14751445]
Pharmacol Biochem Behav. 2004 Feb;77(2):191-7.
In contrast to vertebrates the involvement of glutamate and N-methyl-D-aspartate (NMDA) receptors in brain functions in insects is both poorly understood and somewhat controversial. Here, we have examined the behavioural effects of two noncompetitive NMDA receptor antagonists, memantine (low affinity) and MK-801 (high affinity), on learning and memory in honeybees (Apis mellifera) using the olfactory conditioning of the proboscis extension reflex (PER). We induced memory deficit by injecting harnessed individuals with a glutamate transporter inhibitor, L-trans-2,4-PDC (L-trans-2,4-pyrrolidine dicarboxylate), that impairs long-term (24 h), but not short-term (1 h), memory in honeybees. We show that L-trans-2,4-PDC-induced amnesia is 'rescued' by memantine injected either before training, or before testing, suggesting that memantine restores memory recall rather than memory formation or storage. When injected alone memantine has a mild facilitating effect on memory. The effects of MK-801 are similar to those of L-trans-2,4-PDC. Both pretraining and pretesting injections lead to an impairment of long-term (24 h) memory, but have no effect on short-term (1 h) memory of an olfactory task. The implications of our results for memory processes in the honeybee are discussed.
A pharmacological analysis of the contractile effects of glutamate on rat and human isolated gut smooth muscle strips.[Pubmed:12616958]
Methods Find Exp Clin Pharmacol. 2002 Dec;24(10):661-8.
Although the contractile effects of glutamate and related excitatory amino acids on gut smooth muscle strips have been demonstrated, the mechanisms, and particularly the physiological importance of that action, remain unknown. In this study, glutamate, aspartate, AMPA, quisqualate, cis-ACPD and (2R,4R)-APDC evoked concentration-dependent contraction of isolated adult rat gastric fundus, with EC50 values of 210 microM, 150 microM, 20 microM, 33 microM, and 2.7 microM and 7.9 microM, respectively. L-SOP (0.1 microM-1.9 mM) did not change the basal tone of the preparations. The maximal contractions evoked by glutamate (20 mM) were 38.83% compared with those elicited by acetylcholine (20 microM). The glutamate-evoked contractions were not affected by atropine, verapamil and nicardipine, blocked by CNQX (0.01 microM), or potentiated by Mg2+ (0.01-100 microM), ketamine (0.01-100 microM) and DL-AP5 (0.1-100 microM), as well as L-trans-2,4-PDC (1-100 microM). Analysis of glutamate's action on rat rectum (EC50 = 44 microM) could only be carried out at the early stages, as half of the preparations were not affected by glutamate. Only 5 out of 26 human longitudinal and circular smooth muscle preparations taken from the stomach and three segments of the large intestine were very slightly contracted by glutamate, excluding further analysis. The contractile effects of glutamate on rat gut smooth muscles were mediated by multiple GluR (non-NMDA > NMDA > group I/II mGluRs) located primarily on smooth muscle cells but functional GluRs on neurons and/or nerve fibers of myenteric nervous plexuses could not be excluded. To fully understand the physiological significance of glutamate-evoked contractions in the gut, more research is required, most likely using many different methodological approaches.
Methylation of L-trans-2,4-pyrrolidine dicarboxylate converts the glutamate transport inhibitor from a substrate to a non-substrate inhibitor.[Pubmed:12213465]
Bioorg Med Chem. 2002 Nov;10(11):3509-15.
The 4-methyl analogue of the potent inhibitor of CNS L-glutamate neurotransmitter transporters, L-trans-2,4-PDC, was synthesized via a 1,3-dipolar cycloaddition reaction sequence. The bioassays performed not only exhibit increased potency of the methylated derivative over L-trans-2,4-PDC, but also exhibit non-substrate properties at the rat forebrain synaptosomal glutamate transporter while the parent L-trans-2,4-PDC exhibits substrate properties. These results support two hypotheses developed for distinguishing the physiological properties of transport inhibitors based on molecular modeling studies, and are reported here.
Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal.[Pubmed:11752061]
J Neurochem. 2001 Dec;79(6):1207-16.
Na(+)-dependent excitatory amino acid transporters (EAATs) normally function to remove extracellular glutamate from brain extracellular space, but EAATs can also increase extracellular glutamate by reversal of uptake. Effects of inhibitors on EAATs can be complex, depending on cell type, whether conditions favor glutamate uptake or uptake reversal and whether the inhibitor itself is a substrate for the transporters. The present study assessed EAAT inhibitors for their ability to inhibit glutamate uptake, act as transporter substrates and block uptake reversal in astrocyte and neuron cultures. L-threo-beta-hydroxyaspartate (L-TBHA), DL-threo-beta-benzyloxyaspartate (DL-TBOA), L-trans-pyrrolidine-2,4-dicarboxylic acid (L-trans-2,4-PDC) (+/-)-cis-4-methy-trans-pyrrolidine-2,4-dicarboxylic acid (cis-4-methy-trans-2,4-PDC) and L-antiendo-3,4-methanopyrrolidine-2,4-dicarboxylic acid (L-antiendo-3,4-MPDC) inhibited L-[14C]glutamate uptake in astrocytes with equilibrium binding constants ranging from 17 microM (DL-TBOA and L-TBHA) - 43 microM (cis-4-methy-trans-2,4-PDC). Transportability of inhibitors was assessed in astrocytes and neurons. While L-TBHA, L-trans-2,4-PDC, cis-4-methy-trans-2,4-PDC and L-antiendo-3,4-MPDC displayed significant transporter substrate activities in neurons and astrocytes, DL-TBOA was a substrate only in astrocytes. This effect of DL-TBOA was concentration-dependent, leading to complex effects on glutamate uptake reversal. At concentrations low enough to produce minimal DL-TBOA uptake velocity (< or = 10 microM), DL-TBOA blocked uptake reversal in ATP-depleted astrocytes; this blockade was negated at concentrations that drove substantial DL-TBOA uptake (> 10 microM). These findings indicate that the net effects of EAAT inhibitors can vary with cell type and exposure conditions.
Transient increase in the high affinity [3H]-L-glutamate uptake activity during in vitro development of hippocampal neurons in culture.[Pubmed:11137623]
Neurochem Int. 2001 Apr;38(4):293-301.
The glial GLAST and GLT-1 glutamate transporters are transiently expressed in hippocampal neurons as shown by immunocytochemistry (Plachez et al., 2000. J. Neurosci. Res., 59, 587-593). In order to test if this transient expression is associated to a transient glutamate uptake activity, [3H]-glutamate uptake was studied during the in vitro development of embryonic hippocampal neurons cultured in a defined (serum free) medium. In these cultures, the ratio of the number of glial cells to the number of neurons increased from 1.7 to 11.3% during the first 10 days of culture, while 77% of the neurons died. The number of neurons then remains stable up to 23 days of culture. The initial glutamate uptake velocity at 20 and 200 microM [3H]-glutamate usually increased about five times between 1 and 10 days in vitro (DIV). Interestingly, at 2 microM [3H]-glutamate, the uptake initial velocity showed a biphasic pattern, with a transient peak between 1 and 6 DIV, the maximum being reached at 2 DIV and a delayed regular increase from 8 to 23 DIV. The concentration-dependent curves were best fitted with two saturable sites high and low affinities, at both 2 and 10 DIV. To pharmacologically characterize the transient increased glutamate uptake activity, four uptake inhibitors, L-threo-3-hydroxy-aspartic acid (THA), L-trans-pyrrolidine-2,4-dicarboxylic acid (L-trans-2,4-PDC), dihydrokainate (DHK), and DL-threo-beta-benzyloxyaspartate (TBOA) were tested. THA, L-trans-2,4-PDC and DL-TBOA inhibited glutamate uptake both at 2 and 10 DIV, while the GLT-1 selective uptake inhibitor DHK neither strongly affected the uptake at 2, nor at 10 DIV. These data indicated that, besides the regular increase in the glial-dependent glutamate uptake activity, a transient high-affinity, DHK insensitive, glutamate transport activity in hippocampal neurons in culture is present. This latter activity could potentially be related to the transient expression of the glial GLAST transporter in neurons.
Pharmacological interference with glutamate re-uptake impairs long-term memory in the honeybee, apis mellifera.[Pubmed:10996407]
Behav Brain Res. 2000 Oct;115(1):49-53.
The role of glutamate in the central nervous system of invertebrates is poorly understood. In the present study we examined the effects of a glutamate transporter inhibitor, L-trans-2,4-pyrrolidine dicarboxylate (L-trans-2,4-PDC), on memory formation in the honeybee following a three-trial classical conditioning of the proboscis extension reflex (PER). Pre-training injections of the drug have no effect on acquisition and short-term (1 h) memory, but impair long-term (24 h), associative olfactory memory in a dose-dependent manner. This effect is transient and the amnesiac individuals can be re-trained successfully 48 h after injections. Our results suggest that glutamatergic neurons in the honeybee brain, in particular those found in the mushroom bodies (MBs), may be part of the circuitry involved in processing of long-term olfactory memory. Such a role for this neurotransmitter is consistent with our previous results showing that glutamate and glutamate transporter(s) are localised in regions of the honeybee brain implicated in higher order processing.
Effects of glutamate uptake blockers on stimulus-induced field potentials in rat entorhinal cortex in vitro.[Pubmed:10025568]
Neurosci Lett. 1999 Jan 8;259(2):103-6.
L-Glutamic acid (Glu) is a key excitatory transmitter in the central nervous system. Excessive amounts of Glu are highly toxic to neurons and particularly the entorhinal cortex (EC) exhibits a remarkable loss of cells in the superficial layers in acute brain injury. The accumulation of Glu is limited by a family of high-affinity Glu transporters. Using extracellular potential recordings in rat brain slices we tested whether application of the Glu uptake blockers dihydrokainate and L-trans-pyrrolidine-2,4-dicarboxylate (L-trans-2,4-PDC) affect stimulus-induced field potentials (FPs) in superficial layer III and deep layer V of the medial EC. We found that a high concentration (400 microM) of the uptake blockers significantly reduces stimulus-induced FPs in both layers. At lower concentration (200 microM), only dihydrokainate is efficient. The data show that Glu uptake is involved in the control of extracellular Glu levels during synaptic excitation of layers III and V of the medial EC.
A novel kainate receptor ligand [3H]-(2S,4R)-4-methylglutamate: pharmacological characterization in rabbit brain membranes.[Pubmed:9517418]
Neuropharmacology. 1997 Nov-Dec;36(11-12):1483-8.
Since kainate evokes large non-desensitizing currents at alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, kainate is of limited use in discriminating between AMPA and kainate receptors. Following recent reports that (2S,4R)-4-methylglutamate is a kainate receptor-selective agonist, we have radiolabelled and subsequently characterized the binding of [3H]-(2S,4R)-4-methylglutamate to rabbit whole-brain membranes. [3H]-(2S,4R)-4-methylglutamate binding was rapid, reversible and labelled two sites (KD1 = 3.67+/-0.50 nM/Bmax1 = 0.54+/-0.03 pmol/mg protein and KD2 = 281.66+/-12.33 nM/ Bmax2 = 1.77+/-0.09 pmol/mg protein). [3H]-(2S,4R)-4-methylglutamate binding was displaced by several non-NMDA receptor ligands: domoate > kainate >> L-quisqualate > or = L-glutamate > 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) >> (S)-AMPA = (S)-5-fluorowillardiine > NMDA. Neither the metabotropic glutamate receptor agonists (1S,3R)-ACPD or L-AP4, together with the L-glutamate uptake inhibitor L-trans-2,4-PDC, influenced binding when tested at 100 microM. We conclude that [3H]-(2S,4R)-4-methylglutamate is a useful radioligand for labelling kainate receptors. It possesses high selectivity, and possesses a pharmacology similar to that for rat cloned low-affinity (Glu5 and 6) kainate receptor subunits.
Modulation of glutamate release from rat hippocampal synaptosomes by nitric oxide.[Pubmed:9441904]
Nitric Oxide. 1997 Aug;1(4):315-29.
We used hippocampal synaptosomes to study the effect of NO originating from NO donors and from the activation of the NO synthase on the Ca2+-dependent release of glutamate due to 4-aminopyridine (4-AP) depolarization. We distinguished between the effects of NO on the exocytotic and on the carrier-mediated release of glutamate, which we found to be related to an increase in cGMP content and to a reduction of the ATP/ADP ratio, respectively. The NO donor hydroxylamine, at concentrations < or = 0.3 mM, inhibited the Ca2+-dependent glutamate release evoked by 4-AP, and addition of the NO donor, NOC-7, had a similar effect, which was reversed by the NO scavenger, carboxy-PTIO. Increasing the activity of NO synthase by addition of L-arginine also led to a decrease in the Ca2+-dependent release of glutamate induced by 4-AP, and this effect was reversed by inhibiting NO synthase with NG-nitro-L-arginine. This depression of the exocytotic release of glutamate was accompanied by an increase in cGMP levels due to the stimulation of soluble guanylyl cyclase by NO, produced either by the NO donors (hydroxylamine <0.3 mM) or by the endogenous NO synthase, but no significant decrease in ATP/ADP ratio was observed. However, at concentrations > or = 0.3 mM, hydroxylamine drastically increased the basal release and completely inhibited the Ca2+-dependent release of glutamate (IC50 = 168 microM). At these higher levels of NO, cGMP levels dropped to about 40% of the maximal values obtained at lower concentrations, and the ATP/ADP ratio decreased to about 50% (at 0.3 mM hydroxylamine). The large increase in the basal release could be partially inhibited by L-trans-2,4-PDC, previously loaded into the synaptosomes, suggesting that the nonexocytotic basal release occurred by reversal of the glutamate carrier. Therefore, the increase in cGMP induced by NO stimulation of the guanylyl cyclase decreases the exocytotic release of glutamate, but higher NO levels reduce the ATP/ADP ratio by inhibiting mitochondrial function, which therefore causes the massive release of cytosolic glutamate through the glutamate carrier.
Respiratory effects produced by microinjection of L-glutamate and an uptake inhibitor of L-glutamate into the caudal subretrofacial area of the medulla.[Pubmed:8566094]
Eur J Pharmacol. 1995 Jul 14;280(3):257-75.
The purposes of our study were to determine the type of respiratory changes that would occur when either an excitatory amino acid receptor agonist or an uptake inhibitor was administered into the caudal subretrofacial area. This was done by microinjecting either L-glutamate or L-pyrrolidine-2,4-dicarboxylate (L-trans-2,4-PDC) into the caudal subretrofacial area while monitoring tidal volume, respiratory rate, mean arterial blood pressure and heart rate. Bilateral microinjection of 2.5 nmol of L-glutamate into the caudal subretrofacial area produced apnea in eight of eight animals tested, and the duration of apnea was 27 +/- 2 s. To determine the type of L-glutamate receptor responsible for mediating the apneic response, antagonists of the N-methyl-D-aspartate (NMDA) and non-NMDA receptor, namely, 3-[(RS)-carboxypiperazin-4-yl]-propyl-phosphonic acid (CPP), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), respectively, were tested. Neither antagonist in doses that blocked NMDA (in the case of CPP) and amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) (in the case of CNQX) blocked apnea elicited by L-glutamate. In addition, kynurenic acid, an antagonist of NMDA and non-NMDA ionotropic receptors, failed to block the effect of L-glutamate. Microinjection of the metabotropic receptor agonist drug, trans-L-1-amino-1,3-cyclopentone-dicarboxylic acid (L-trans-ACPD), into the caudal subretrofacial area failed to have any effect on respiratory activity. Because of the inability to block the effect of L-glutamate in the caudal subretrofacial area, and the lack of effect of L-trans-ACPD, the data suggest that the apneic response produced by L-glutamate is mediated by an as yet undefined receptor. Microinjection of the L-glutamate uptake inhibitor, L-trans-2,4-PDC, was found to produce apnea. Using the dose of 0.5 nmol of L-trans-2,4-PDC, we examined the type of excitatory amino acid receptor that mediated the response. Neither pretreatment with the NMDA receptor antagonist, CPP, nor the non-NMDA receptor antagonist, CNQX, affected L-trans-2,4-PDC-induced apnea. However, combined use of these two antagonists prevented L-trans-2,4-PDC-induced apnea. These data suggest that the effect of synaptically released exitatory amino acid at the caudal subretrofacial area on breathing is apnea, and that this effect is mediated by simultaneous activation of both NMDA and non-NMDA ionotropic receptors.
Pharmacological dissociation of glutamatergic metabotropic signal transduction pathways in cortical astrocytes.[Pubmed:7851499]
Eur J Pharmacol. 1994 Oct 14;269(2):235-41.
Using cultured cortical astrocytes we demonstrate differential activation of metabotropic signal transduction pathways with 1-aminocyclopentane-trans-1S3R-dicarboxylic acid (1S3R-ACPD) and the glutamate transport inhibitor trans-2,4-pyrrolidine dicarboxylic acid (trans-2,4-PDC). Phosphoinositide hydrolysis was more potently stimulated by 1S3R-ACPD than by L-trans-2,4-PDC; however, L-trans-2,4-PDC was far more efficacious than 1S3R-ACPD at inhibiting cyclic AMP accumulation. The metabotropic receptor antagonist (+)-alpha-methyl-4-carboxyphenylglycine ((+)-MCPG) inhibited 1S3R-ACPD stimulation of phosphoinositide hydrolysis but not its ability to inhibit cyclic AMP accumulation thereby demonstrating a means to pharmacologically dissociate these two metabotropic signal transduction pathways in astrocytes. (+)-MCPG produced similar antagonism of the metabotropic agonist properties of L-trans-2,4-PDC. The metabotropic effects of L-trans-2,4-PDC could not be reduced with enzymatic treatment of the cultures to remove extracellular glutamate, suggesting that these effects are not secondary to the ability of this compound to inhibit glutamate uptake. Taken together the findings indicate the presence of multiple glutamatergic signal transduction pathways in astrocytes and suggest a similarity in the pharmacophores for metabotropic receptors and glutamate transporters.
Effects of uptake carrier blockers SK & F 89976-A and L-trans-PDC on in vivo release of amino acids in rat hippocampus.[Pubmed:8836615]
Eur J Pharmacol. 1996 Jul 4;307(3):275-82.
This report describes the in vivo effects of the uptake carrier blockers 1-(4,4-diphenyl-3-butenyl)-3-piperidine carboxylic acid hydrochloride (SK & F 89976-A) and L-trans-pyrrolidine-2,4-dicarboxylate (L-trans-PDC) on basal and K(+)-evoked extracellular levels of gamma-aminobutyric acid (GABA), glutamate, aspartate and taurine in the hippocampus of anaesthetised rats, using the microdialysis technique. SK & F 89976-A increased extracellular GABA levels under K(+)-depolarised conditions and did not affect extracellular glutamate, aspartate and taurine levels, indicating its selective effect on GABA uptake L-trans-PDC dose dependently increased basal and K(+)-evoked extracellular glutamate levels, and did not affect extracellular GABA levels, but increased basal aspartate and taurine levels. The K(+)-evoked release of GABA and glutamate, measured in the presence of both SK & F 89976-A and L-trans-PDC, was Ca(2+)-dependent for about 50% and 65%, respectively. In contrast, the release of the putative amino acid transmitters aspartate and taurine was not Ca(2+)-dependent. These results indicate that (1) in rat hippocampus uptake carriers actively regulate extracellular GABA and glutamate levels, (2) the GABA and glutamate released by K+ was derived from both Ca(2+)-dependent (presumably vesicular) and Ca(2+)-independent (presumably cytosolic) pools, whereas aspartate and taurine release was exclusively from Ca(2+)-independent pools.
Regional differences in the inhibition of L-glutamate and L-aspartate sodium-dependent high affinity uptake systems in rat CNS synaptosomes by L-trans-pyrrolidine-2,4-dicarboxylate, threo-3-hydroxy-D-aspartate and D-aspartate.[Pubmed:7981641]
Neurochem Int. 1994 Jun;24(6):583-8.
The sodium-dependent high affinity transport of L-[3H]glutamate and L-[3H]aspartate into synaptosomal fractions prepared from three different regions was employed to investigate the inhibitors L-trans-pyrrolidine-2,4-dicarboxylate, threo-3-hydroxy-D-aspartate and D-aspartate. These substances showed regional heterogeneity as inhibitors of sodium-dependent high affinity uptake of L-glutamate and L-aspartate. L-trans-Pyrrolidine-2,4-dicarboxylate was a more potent inhibitor of the uptake of L-glutamate than of L-aspartate in the cortex (IC50 8 microM vs L-glutamate and 13 microM vs L-aspartate) and cerebellum (IC50 4 microM v L-glutamate and 8 microM vs L-aspartate). threo-3-Hydroxy-D-aspartate was a more potent inhibitor of the uptake of L-glutamate than of L-aspartate in the cortex (IC50 9 microM vs L-glutamate and 13 microM vs L-aspartate) and hippocampus (IC50 6 microM v L-glutamate and 11 microM v L-aspartate). D-Aspartate was a more potent inhibitor of the uptake of L-glutamate than of L-aspartate only in the cortex (IC50 8 microM vs L-glutamate and 15 microM vs L-aspartate). These results thus support other evidence that there is regional heterogeneity in sodium-dependent high affinity acidic amino acid uptake sites in the brain.
Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2,4-dicarboxylate diastereomer.[Pubmed:1671706]
J Med Chem. 1991 Feb;34(2):717-25.
In order to determine the conformational requirements for binding of L-glutamate to the proteins involved in the process of neurotransmission, rigid analogues containing an embedded glutamate moiety have been prepared. These "conformer mimics", the pyrrolidine-2,4-dicarboxylates 4, 7, 11, and 14, were synthesized from commercially available trans-4-hydroxy-L-proline and cis-4-hydroxy-D-proline, and then were tested for their ability to inhibit the high-affinity transport of [3H]-L-glutamate into synaptosomes and to block the binding of radioligands to the NMDA (N-methyl-D-aspartate), KA (kainate), and QA (quisqualate) glutamate neurotransmitter receptor sites. While none of the four analogues binds effectively to the excitatory receptors, the L-trans-isomer 7 is a potent and selective competitive inhibitor of L-glutamate transport. These results delineate a specific structural/conformational preference for binding to the uptake system that is distinct from that required for binding to the NMDA, KA, and QA receptors.


