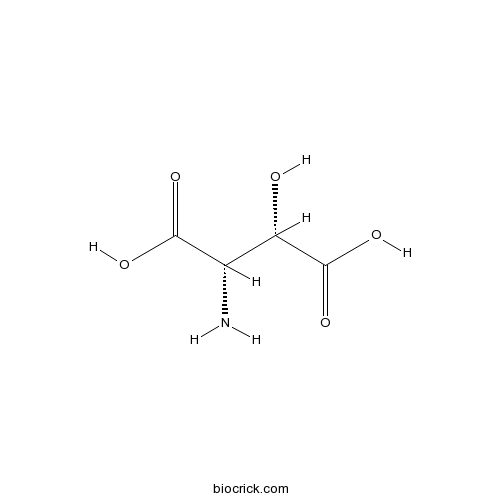
L-(-)-threo-3-Hydroxyaspartic acidTransportable EAAT1-4 inhibitor/non-transportable EAAT5 inhibitor CAS# 7298-99-9 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 7298-99-9 | SDF | Download SDF |
| PubChem ID | 443239 | Appearance | Powder |
| Formula | C4H7NO5 | M.Wt | 149.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L-<em>threo</em>-β-Hydroxyaspartic acid | ||
| Solubility | Soluble to 100 mM in 1eq. NaOH | ||
| Chemical Name | (2S,3S)-2-amino-3-hydroxybutanedioic acid | ||
| SMILES | C(C(C(=O)O)O)(C(=O)O)N | ||
| Standard InChIKey | YYLQUHNPNCGKJQ-LWMBPPNESA-N | ||
| Standard InChI | InChI=1S/C4H7NO5/c5-1(3(7)8)2(6)4(9)10/h1-2,6H,5H2,(H,7,8)(H,9,10)/t1-,2-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, competitive, transportable EAAT1-4 inhibitor/non-transportable EAAT5 inhibitor. Also available as part of the Excitatory Amino Acid Transporter Inhibitor. |

L-(-)-threo-3-Hydroxyaspartic acid Dilution Calculator

L-(-)-threo-3-Hydroxyaspartic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7069 mL | 33.5345 mL | 67.0691 mL | 134.1382 mL | 167.6727 mL |
| 5 mM | 1.3414 mL | 6.7069 mL | 13.4138 mL | 26.8276 mL | 33.5345 mL |
| 10 mM | 0.6707 mL | 3.3535 mL | 6.7069 mL | 13.4138 mL | 16.7673 mL |
| 50 mM | 0.1341 mL | 0.6707 mL | 1.3414 mL | 2.6828 mL | 3.3535 mL |
| 100 mM | 0.0671 mL | 0.3353 mL | 0.6707 mL | 1.3414 mL | 1.6767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- Gomisin O
Catalog No.:BCN2875
CAS No.:72960-22-6
- Gomisin E
Catalog No.:BCN7031
CAS No.:72960-21-5
- 6,7-Dihydroneridienone A
Catalog No.:BCN4020
CAS No.:72959-46-7
- 2-Furoyl-LIGRLO-amide
Catalog No.:BCC3958
CAS No.:729589-58-6
- Carvedilol
Catalog No.:BCC4324
CAS No.:72956-09-3
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
- 30-Hydroxylup-20(29)-en-3-one
Catalog No.:BCN4286
CAS No.:72944-06-0
- Octadecyl p-coumarate
Catalog No.:BCN7235
CAS No.:72943-88-5
- K858
Catalog No.:BCC7760
CAS No.:72926-24-0
- Cordycepin
Catalog No.:BCN5389
CAS No.:73-03-0
- H-Trp-OH
Catalog No.:BCC3111
CAS No.:73-22-3
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
One-Pot Production of L-threo-3-Hydroxyaspartic Acid Using Asparaginase-Deficient Escherichia coli Expressing Asparagine Hydroxylase of Streptomyces coelicolor A3(2).[Pubmed:25795668]
Appl Environ Microbiol. 2015 Jun;81(11):3648-54.
We developed a novel process for efficient synthesis of L-threo-3-hydroxyaspartic acid (L-THA) using microbial hydroxylase and hydrolase. A well-characterized mutant of asparagine hydroxylase (AsnO-D241N) and its homologous enzyme (SCO2693-D246N) were adaptable to the direct hydroxylation of L-aspartic acid; however, the yields were strictly low. Therefore, the highly stable and efficient wild-type asparagine hydroxylases AsnO and SCO2693 were employed to synthesize L-THA. By using these recombinant enzymes, L-THA was obtained by L-asparagine hydroxylation by AsnO followed by amide hydrolysis by asparaginase via 3-hydroxyasparagine. Subsequently, the two-step reaction was adapted to one-pot bioconversion in a test tube. L-THA was obtained in a small amount with a molar yield of 0.076% by using intact Escherichia coli expressing the asnO gene, and thus, two asparaginase-deficient mutants of E. coli were investigated. A remarkably increased L-THA yield of 8.2% was obtained with the asparaginase I-deficient mutant. When the expression level of the asnO gene was enhanced by using the T7 promoter in E. coli instead of the lac promoter, the L-THA yield was significantly increased to 92%. By using a combination of the E. coli asparaginase I-deficient mutant and the T7 expression system, a whole-cell reaction in a jar fermentor was conducted, and consequently, L-THA was successfully obtained from L-asparagine with a maximum yield of 96% in less time than with test tube-scale production. These results indicate that asparagine hydroxylation followed by hydrolysis would be applicable to the efficient production of L-THA.
(2S,3S,4R)-2-(carboxycyclopropyl)glycine, a potent and competitive inhibitor of both glial and neuronal uptake of glutamate.[Pubmed:7901789]
Neuropharmacology. 1993 Sep;32(9):833-7.
The effects of several diastereoisomers of L-2-(carboxycyclopropyl)glycine (CCG) on L-glutamate uptake were compared among three different preparations, glial plasmalemmal vesicles (GPV), synaptosomes and cultured astrocytes from rat hippocampus. The (2S,3S,4R)-isomer (L-CCG-III) inhibited a Na(+)-dependent high-affinity L-glutamate uptake in GPV and synaptosomes in a dose dependent manner at a micromolar range. The potency was quite similar to that of L-threo-beta-hydroxyaspartate in both subcellular fractions and much higher than L-aspartate-beta-hydroxamate, which were known as potent inhibitors of glutamate uptake. The (2S,3R,4S)-isomer (L-CCG-IV) also inhibited the glutamate uptake in GPV and synaptosomes, but it was about 100 times less active than L-CCG-III. The (2S,3S,4S)- and (2S,3R,4R)-isomers (L-CCG-I and L-CCG-II, respectively) hardly showed any inhibitory action on the glutamate uptake. Dixon plot analysis of the initial uptake rate revealed that the inhibition was in a competitive manner and the value of the inhibition constant (Ki) was about 1 microM in both GPV and synaptosomes. L-CCG-III effectively inhibited the glutamate uptake by cultured hippocampal astrocytes as well. These results suggested that L-CCG-III inhibited the glutamate uptake in both neurones and glial cells of the mammalian central nervous system in a similar manner.
Neurotoxicity of L-glutamate and DL-threo-3-hydroxyaspartate in the rat striatum.[Pubmed:2856883]
J Neurochem. 1985 Jan;44(1):247-54.
Destruction of the glutamatergic corticostriatal pathway potentiates the neurotoxic action of 1 mumol L-glutamate injected into the rat striatum, whereas the toxic effects of 10 nmol kainate are markedly attenuated. Injection of 170 nmol of the glutamate uptake inhibitor, DL-threo-3-hydroxyaspartate, into the intact striatum also causes neuronal degeneration, which is accompanied by a reduction in markers for cholinergic and GABAergic neurones. Prior removal of the corticostriatal pathway destroys the ability of DL-threo-3-hydroxyaspartate to cause lesions in the striatum. These results indicate that removal, or blockade, of uptake sites for glutamate increase the vulnerability of striatal neurones to the toxic effects of synaptically released glutamate.


