IstaroximeNa+/K+ ATPase inhibitor CAS# 203737-93-3 |
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
Number of papers citing our products

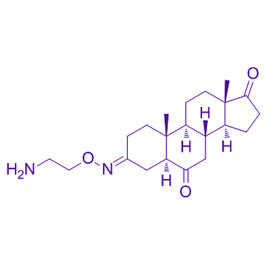
Chemical structure

3D structure
| Cas No. | 203737-93-3 | SDF | Download SDF |
| PubChem ID | 9841834 | Appearance | Powder |
| Formula | C21H32N2O3 | M.Wt | 360.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PST2744 | ||
| Solubility | 25℃: 10 mM in distilled water | ||
| Chemical Name | (3E,5S,8R,9S,10R,13S,14S)-3-(2-aminoethoxyimino)-10,13-dimethyl-1,2,4,5,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthrene-6,17-dione | ||
| SMILES | CC12CCC(=NOCCN)CC1C(=O)CC3C2CCC4(C3CCC4=O)C | ||
| Standard InChIKey | MPYLDWFDPHRTEG-PAAYLBSLSA-N | ||
| Standard InChI | InChI=1S/C21H32N2O3/c1-20-7-5-13(23-26-10-9-22)11-17(20)18(24)12-14-15-3-4-19(25)21(15,2)8-6-16(14)20/h14-17H,3-12,22H2,1-2H3/b23-13+/t14-,15-,16-,17+,20+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Istaroxime is a potent inhibitor of Na+,K+-ATPase with IC50 of 0.11 μM.In Vitro:Istaroxime acting as a positive inotropic compound through the inhibition of the Na+,K+-ATPase[2]. Istaroxime (PST2744) inhibits the Na+/K+-ATPase activity from dog kidney with an IC50 value of 0.43 ± 0.15 μM. Inhibition of Na+/K+-ATPase activity in preparations from guinea pig kidney yielded potencies of 8.5 μM for PST2744[3].In Vivo:Istaroxime (PST2744) induces a progressive increase in +dP/dtmax throughout the infusion that reaches 80% (ED80) at the cumulative dose of 1.89±0.37 mg/kg and a peak of 140±3.5% at the dose (EDmax) of 4.88±0.6 mg/kg[3]. References: | |||||

Istaroxime Dilution Calculator

Istaroxime Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.774 mL | 13.87 mL | 27.74 mL | 55.48 mL | 69.3501 mL |
| 5 mM | 0.5548 mL | 2.774 mL | 5.548 mL | 11.096 mL | 13.87 mL |
| 10 mM | 0.2774 mL | 1.387 mL | 2.774 mL | 5.548 mL | 6.935 mL |
| 50 mM | 0.0555 mL | 0.2774 mL | 0.5548 mL | 1.1096 mL | 1.387 mL |
| 100 mM | 0.0277 mL | 0.1387 mL | 0.2774 mL | 0.5548 mL | 0.6935 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.43 ± 0.15 μM (Na+/K+-ATPase activity from dog kidney) [1] Istaroxime is a positive inotropic agent that mediates its action through inhibition of sodium/potassium adenosine triphosphatase (Na+/K+ ATPase). Istaroxime is an investigational drug originally patented and developed by the italian pharmaceutical company Sigma-Tau. Istaroxime is now under development for treatment of acute decompensated heart failure by CVie Therapeutics. in vitro: PST2744 inhibited the Na+/K+-ATPase activity from dog kidney with an IC50 value of 0.43 ± 0.15 μM [1]. The transient inward current (I(TI)) induced by a Ca(2+) transient in the presence of complete Na(+)/K(+) pump blockade was inhibited (-43%) by PST2744 but not by digoxin [2]. in vivo: Intravenous infusion of 0.2 mg/kg/min PST2744 in anesthetized guinea pigs exerted an immediate and long-lasting inotropic effect (ED(80) of 1.89 +/- 0.37 mg/kg) without causing lethal arrhythmias up to a cumulative dose of 18 mg/kg [1]. Istaroxime intravenous infusion (0.11 mg/kg per min) significantly increased both indices of contraction and relaxation (fractional shortening, +18+/-3.7%; aortic flow rate, +19+/-2.9%; peak myocardial systolic velocity, +36+/-7%; circumferential fiber shortening, +24+/-4.1%; peak atrial flow velocity, +69+/-8.6%; isovolumic relaxation time, +19+/-6.9%; and peak myocardial early diastolic velocity, +42+/-12%) [3]. In 5 animals, PST-2744 effects were compared with dobutamine. Heart rates, PR intervals and QT intervals were unchanged following PST-2744 administration. PST-2744 increased contractility (+dP/dt) by 56% from 1881 +/- 282 mm Hg/s to 2939 +/- 734 mm Hg/s (P < 0.01) [4]. Clinical trial: HORIZON-HF: A Phase II Trial to Assess Hemodynamic Effects of Istaroxime in Patients With Worsening HF and Reduced LV Systolic Function. Phase 2
- Arctiin
Catalog No.:BCN1090
CAS No.:20362-31-6
- Hastacine
Catalog No.:BCN2086
CAS No.:20361-77-7
- DMNB
Catalog No.:BCC7259
CAS No.:20357-25-9
- 2,4,6,6-Tetramethyl-3(6H)-pyridinone
Catalog No.:BCN4893
CAS No.:203524-64-5
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- (-)-Maackiain
Catalog No.:BCN4892
CAS No.:2035-15-6
- (+)-Bornyl acetate
Catalog No.:BCN8317
CAS No.:20347-65-3
- SNX 482
Catalog No.:BCC5952
CAS No.:203460-30-4
- 18-Norabieta-8,11,13-triene-4,15-diol
Catalog No.:BCN1504
CAS No.:203455-81-6
- Luteollin 5-glucoside
Catalog No.:BCN5391
CAS No.:20344-46-1
- 7-Oxo-beta-sitosterol
Catalog No.:BCN4891
CAS No.:2034-74-4
- Daphnoretin
Catalog No.:BCN2473
CAS No.:2034-69-7
- 27-Hydroxycholesterol
Catalog No.:BCN2750
CAS No.:20380-11-4
- MMAD
Catalog No.:BCC1774
CAS No.:203849-91-6
- Fmoc-ß-HoGlu(OtBu)-OH
Catalog No.:BCC3234
CAS No.:203854-49-3
- Helioxanthin derivative 5-4-2
Catalog No.:BCC5414
CAS No.:203935-39-1
- SU5416
Catalog No.:BCC1974
CAS No.:204005-46-9
- PD 176252
Catalog No.:BCC7426
CAS No.:204067-01-6
- (2-Aminoethyl)phosphonic acid
Catalog No.:BCN1762
CAS No.:2041-14-7
- Anabasamine
Catalog No.:BCN2148
CAS No.:20410-87-1
- 4'-Hydroxy-2-O-methylanigorufone
Catalog No.:BCN7179
CAS No.:204134-70-3
- Caesalmin E
Catalog No.:BCN7247
CAS No.:204185-91-1
- Boc-Ser-OH.H2O
Catalog No.:BCC2599
CAS No.:204191-40-2
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
Functional characterization and anti-cancer action of the clinical phase II cardiac Na+/K+ ATPase inhibitor istaroxime: in vitro and in vivo properties and cross talk with the membrane androgen receptor.[Pubmed:27027435]
Oncotarget. 2016 Apr 26;7(17):24415-28.
Sodium potassium pump (Na+/K+ ATPase) is a validated pharmacological target for the treatment of various cardiac conditions. Recent published data with Na+/K+ ATPase inhibitors suggest a potent anti-cancer action of these agents in multiple indications. In the present study, we focus on Istaroxime, a Na+/K+ ATPase inhibitor that has shown favorable safety and efficacy properties in cardiac phase II clinical trials. Our experiments in 22 cancer cell lines and in prostate tumors in vivo proved the strong anti-cancer action of this compound. Istaroxime induced apoptosis, affected the key proliferative and apoptotic mediators c-Myc and caspase-3 and modified actin cystoskeleton dynamics and RhoA activity in prostate cancer cells. Interestingly, Istaroxime was capable of binding to mAR, a membrane receptor mediating rapid, non-genomic actions of steroids in prostate and other cells. These results support a multi-level action of Na+/K+ ATPase inhibitors in cancer cells and collectively validate Istaroxime as a strong re-purposing candidate for further cancer drug development.
Istaroxime: A rising star in acute heart failure.[Pubmed:23326115]
J Pharmacol Pharmacother. 2012 Oct;3(4):353-5.
Heart failure in India is a growing epidemic. Around 30 to 40% of patients die from heart failure within one year of receiving the diagnosis. Currently available inotropes have not only failed to show consistent results but are also associated with adverse outcomes. Istaroxime is a novel intravenous agent with luso-inotropic properties that acts by inhibition of Na(+)/K(+) adenosine triphosphatase and stimulation of sarco/ endoplasmic reticulum calcium ATPase isoform 2. In clinical studies, it significantly decreased left ventricular end diastolic pressure, pulmonary capillary wedge pressure, heart rate and increased systolic blood pressure and cardiac index with no change in neurohormones, renal function or troponin I. Istaroxime is a promising alternative for patients presenting with acute heart failure syndrome for whom the therapeutic options are currently limited.
SERCA2a stimulation by istaroxime: a novel mechanism of action with translational implications.[Pubmed:23822610]
Br J Pharmacol. 2013 Oct;170(3):486-8.
UNLABELLED: Sarcoplasmic reticular (SR) Ca(2+) -ATPase (SERCA2a) is central to cardiac electrophysiological and mechanical function. It ensures full diastolic relaxation minimizing delayed after-potentials that would otherwise compromise membrane electrophysiological stability, and optimizes SR Ca(2+) refilling and systolic contraction. Previous studies demonstrated that the small molecule agent Istaroxime stimulates SERCA2a-ATPase activity, restoring its function in failing hearts, and enhancing indices of mechanical, and SR Ca(2+) release and re-uptake, activity. Ferrandi et al (2013) now elegantly demonstrate its ability to dissociate the phospholamdan (PB) bound to cardiac SERCA2a, thereby removing the inhibitory effect of PB on SERCA2a. This effect was independent of the cAMP/PKA system and modified a specific SERCA2a reaction step. They used SERCA-enriched SR preparations from a rigorously validated and realistic physiological, canine model of cardiac failure with established Na(+) -K(+) -ATPase sensitivity to cardiac glycosides and SR Ca(2+) handling features. These findings potentially translate into a novel management of the major and increasingly important public health challenge of chronic cardiac failure. LINKED ARTICLE: This article is a commentary on Ferrandi et al., pp. 1849-1861 of volume 169 issue 8. To view this paper visit http://dx.doi.org/10.1111/bph.12278.
Istaroxime stimulates SERCA2a and accelerates calcium cycling in heart failure by relieving phospholamban inhibition.[Pubmed:23763364]
Br J Pharmacol. 2013 Aug;169(8):1849-61.
BACKGROUND AND PURPOSE: Calcium handling is known to be deranged in heart failure. Interventions aimed at improving cell Ca(2) (+) cycling may represent a promising approach to heart failure therapy. Istaroxime is a new luso-inotropic compound that stimulates cardiac contractility and relaxation in healthy and failing animal models and in patients with acute heart failure (AHF) syndrome. Istaroxime is a Na-K ATPase inhibitor with the unique property of increasing sarcoplasmic reticulum (SR) SERCA2a activity as shown in heart microsomes from humans and guinea pigs. The present study addressed the molecular mechanism by which Istaroxime increases SERCA2a activity. EXPERIMENTAL APPROACH: To study the effect of Istaroxime on SERCA2a-phospholamban (PLB) complex, we applied different methodologies in native dog healthy and failing heart preparations and heterologous canine SERCA2a/PLB co-expressed in Spodoptera frugiperda (Sf21) insect cells. KEY RESULTS: We showed that Istaroxime enhances SERCA2a activity, Ca(2) (+) uptake and the Ca(2) (+) -dependent charge movements into dog healthy and failing cardiac SR vesicles. Although not directly demonstrated, the most probable explanation of these activities is the displacement of PLB from SERCA2a.E2 conformation, independently from cAMP/PKA. We propose that this displacement may favour the SERCA2a conformational transition from E2 to E1, thus resulting in the acceleration of Ca(2) (+) cycling. CONCLUSIONS AND IMPLICATIONS: Istaroxime represents the first example of a small molecule that exerts a luso-inotropic effect in the failing human heart through the stimulation of SERCA2a ATPase activity and the enhancement of Ca(2) (+) uptake into the SR by relieving the PLB inhibitory effect on SERCA2a in a cAMP/PKA independent way.


