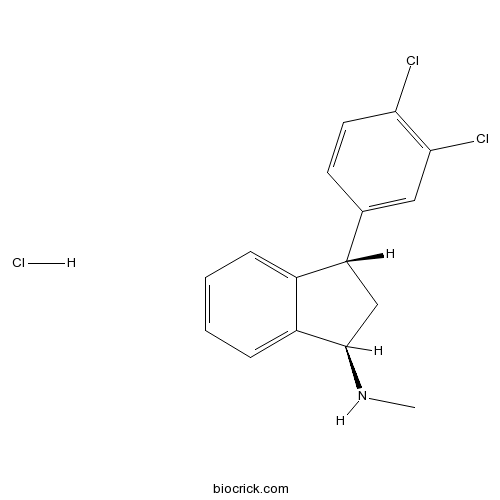
Indatraline hydrochloridePotent 5-HT uptake inhibitor; also inhibits dopamine and noradrenalin uptake CAS# 96850-13-4 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- RS 504393
Catalog No.:BCC1910
CAS No.:300816-15-3
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 96850-13-4 | SDF | Download SDF |
| PubChem ID | 10314472 | Appearance | Powder |
| Formula | C16H16Cl3N | M.Wt | 328.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Lu 19-005 | ||
| Solubility | Soluble to 10 mM in water with gentle warming and to 100 mM in DMSO | ||
| Chemical Name | (1R,3S)-3-(3,4-dichlorophenyl)-N-methyl-2,3-dihydro-1H-inden-1-amine;hydrochloride | ||
| SMILES | CNC1CC(C2=CC=CC=C12)C3=CC(=C(C=C3)Cl)Cl.Cl | ||
| Standard InChIKey | QICQDZXGZOVTEF-MELYUZJYSA-N | ||
| Standard InChI | InChI=1S/C16H15Cl2N.ClH/c1-19-16-9-13(11-4-2-3-5-12(11)16)10-6-7-14(17)15(18)8-10;/h2-8,13,16,19H,9H2,1H3;1H/t13-,16+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent monoamine uptake inhibitor. Inhibits transporters for 5-HT (SERT), dopamine (DAT) and noradrenalin (NET) (Ki values are 0.42, 1.7 and 5.8 nM respectively). Centrally active following systemic administration in vivo. |

Indatraline hydrochloride Dilution Calculator

Indatraline hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0426 mL | 15.2128 mL | 30.4257 mL | 60.8513 mL | 76.0641 mL |
| 5 mM | 0.6085 mL | 3.0426 mL | 6.0851 mL | 12.1703 mL | 15.2128 mL |
| 10 mM | 0.3043 mL | 1.5213 mL | 3.0426 mL | 6.0851 mL | 7.6064 mL |
| 50 mM | 0.0609 mL | 0.3043 mL | 0.6085 mL | 1.217 mL | 1.5213 mL |
| 100 mM | 0.0304 mL | 0.1521 mL | 0.3043 mL | 0.6085 mL | 0.7606 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Przewaquinone C
Catalog No.:BCN3003
CAS No.:96839-29-1
- Orlistat
Catalog No.:BCC3830
CAS No.:96829-58-2
- Chlorovaltrate K
Catalog No.:BCN7126
CAS No.:96801-92-2
- (±)-McN 5652
Catalog No.:BCC7267
CAS No.:96795-89-0
- Chlorisondamine diiodide
Catalog No.:BCC6885
CAS No.:96750-66-2
- [D-Arg1,D-Phe5,D-Trp7,9,Leu11]-Substance P
Catalog No.:BCC7211
CAS No.:96736-12-8
- Rabdoserrin A
Catalog No.:BCN8041
CAS No.:96685-01-7
- Viscidulin III tetraacetate
Catalog No.:BCN4515
CAS No.:96684-81-0
- Decuroside I
Catalog No.:BCN3909
CAS No.:96638-79-8
- Ocinaplon
Catalog No.:BCC6167
CAS No.:96604-21-6
- 3beta-Hydroxylanosta-8,24-diene-21-al
Catalog No.:BCN3329
CAS No.:96574-03-7
- Salvianolic acid A
Catalog No.:BCN5951
CAS No.:96574-01-5
- 8beta-Tigloyloxycostunolide
Catalog No.:BCN7115
CAS No.:96850-21-4
- Maoecrystal B
Catalog No.:BCN4516
CAS No.:96850-29-2
- Maoecrystal A
Catalog No.:BCN5407
CAS No.:96850-30-5
- XCC
Catalog No.:BCC7890
CAS No.:96865-83-7
- XAC
Catalog No.:BCC7600
CAS No.:96865-92-8
- VIP (guinea pig)
Catalog No.:BCC5725
CAS No.:96886-24-7
- Nyasol
Catalog No.:BCN7579
CAS No.:96895-25-9
- 1,2-Bis(4'-methyl-2,2'-bipyridin-4-yl)ethane
Catalog No.:BCC8414
CAS No.:96897-04-0
- Cyproheptadine hydrochloride
Catalog No.:BCC5161
CAS No.:969-33-5
- Artanin
Catalog No.:BCN4517
CAS No.:96917-26-9
- Cisatracurium Besylate
Catalog No.:BCC4345
CAS No.:96946-42-8
- Momordin Ic
Catalog No.:BCN1216
CAS No.:96990-18-0
A cell-based quantitative high-throughput image screening identified novel autophagy modulators.[Pubmed:27168224]
Pharmacol Res. 2016 Aug;110:35-49.
Macroautophagy is a major cellular degradation pathway for long-lived proteins and cellular organelles to maintain cellular homeostasis. Reduced autophagy has been implicated in neurodegenerative diseases, metabolic syndrome, and tumorigenesis. In contrast, increased autophagy has been shown to protect against tissue injury and aging. Here we employed a cell-based quantitative high-throughput image screening (qHTS) for autophagy modulators using mouse embryonic fibroblasts (MEFs) that are stably expressing GFP-LC3. The library of pharmacologically active compounds (LOPAC) was used to screen for the autophagy modulators in compounds alone or in combination with the lysosome inhibitor chloroquine (CQ). The GFP-LC3 puncta were then quantified to measure autophagic flux. The primary screening revealed 173 compounds with efficacy more than 40%. These compounds were cherry-picked and re-tested at multiple different concentrations using the same assay. A number of novel autophagy inducers, inhibitors, and modulators with dual-effects on autophagy were identified from the cherry-pick screening. Interestingly, we found a group of compounds that induce autophagy are related to dopamine receptors and are commonly used as clinical psychiatric drugs. Among them, Indatraline hydrochloride (IND), a dopamine inhibitor, and chlorpromazine hydrochloride (CPZ) and fluphenazine dihydrochloride (FPZ), two dopamine receptor antagonists, were further evaluated. We found that FPZ-induced autophagy through mTOR inhibition but IND and CPZ induced autophagy in an mTOR-independent manner. Our data suggest that image-based autophagic flux qHTS can efficiently identify autophagy inducers and inhibitors.
Design, synthesis, and monoamine transporter binding site affinities of methoxy derivatives of indatraline.[Pubmed:11123996]
J Med Chem. 2000 Dec 14;43(25):4868-76.
A series of methoxy-containing derivatives of indatraline 13a-f and 17 were synthesized, and their binding affinities for the dopamine, serotonin, and norepinephrine transporter binding sites were determined. Introduction of a methoxy group to indatraline affected its affinity and selectivity greatly. Except for the 4-methoxy derivative 13a,which had the same high affinity at the dopamine transporter binding site as indatraline, the other methoxy-containing analogues (13b-f and 17) exhibited lower affinity than indatraline for the three transporter binding sites. However, some of the analogues were more selective than indatraline, and the 6-methoxy derivative 13c displayed the highest affinity for both the serotonin and norepinephrine transporters. This compound retained reasonable affinity for the dopamine transporter and is a promising template for the development of a long-acting inhibitor of monoamine transporters. Such inhibitors have potential as medications for treatment, as a substitution medication, or for prevention of the abuse of methamphetamine-like stimulants.
Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys.[Pubmed:10490887]
J Pharmacol Exp Ther. 1999 Oct;291(1):60-9.
Cocaine is a nonselective monoamine reuptake inhibitor that is widely abused. Useful pharmacotherapies for cocaine dependence may include substitution medications that produce cocaine-like effects but have a slower onset and longer duration of action. Accordingly, the present study examined the effects of the long-acting, nonselective monoamine reuptake inhibitor indatraline in assays of cocaine discrimination and cocaine self-administration that have been used to evaluate other candidate treatment medications. In rhesus monkeys trained to discriminate cocaine (0.4 mg/kg) from saline, indatraline (0.1-1.0 mg/kg) produced a dose- and time-dependent substitution for cocaine. The effects of 1.0 mg/kg indatraline peaked after 30 min and lasted up to 24 h. In monkeys trained to self-administer 0.032 mg/kg/injection cocaine and food pellets during alternating daily sessions of cocaine and food availability, indatraline (0.0032-0.032 mg/kg/injection) maintained lower rates of responding than cocaine. Repeated treatments with indatraline (0.1-0.56 mg/kg/day) for 7 days produced dose-dependent and sustained decreases in cocaine self-administration across a broad range of cocaine doses (0.0032-0.1 mg/kg/injection), and the highest dose of indatraline (0.56 mg/kg/day) nearly eliminated cocaine-maintained responding. However, doses of indatraline that decreased cocaine self-administration also usually decreased rates of food-maintained responding and produced behavioral stereotypies and trends toward weight loss and mild anemia. These findings suggest that although indatraline may decrease cocaine-taking behavior in rhesus monkeys, it also produces undesirable side effects that may limit its clinical utility in the treatment of cocaine dependence.
Neurochemical profile of Lu 19-005, a potent inhibitor of uptake of dopamine, noradrenaline, and serotonin.[Pubmed:2580950]
J Neurochem. 1985 May;44(5):1615-22.
The neurochemical profile of a new compound, Lu 19-005 [(+/-)trans-3-(3,4-dichlorophenyl)-N-methyl-1-indanamine hydrochloride], has been investigated. Lu 19-005 is a potent inhibitor of the synaptosomal uptake of 3,4-dihydroxyphenylethylamine (dopamine, DA), noradrenaline (NA), and 5-hydroxytryptamine (5-HT, serotonin). In this respect it resembles diclofensine, whereas compounds such as GBR 13.069 and bupropion are more selective DA-uptake inhibitors. Although Lu 19-005 releases DA in in higher concentrations it must be considered as an uptake inhibitor, as the accumulation of DA is inhibited in much lower concentrations. Lu 19-005 attenuates the DA and NA depletion caused by 6-hydroxydopamine in mouse brain. These properties confirm the DA- and NA-uptake-inhibitory properties of the compound. In receptor-binding models and functional in vitro tests Lu 19-005 is devoid of dopaminergic-, serotonergic-, noradrenergic-, histaminergic-, and cholinergic-inhibiting properties. Since DA, NA, and 5-HT seem to be involved in depression, the profile of Lu 19-005--with equally potent activity on the three neuronal systems--makes it an interesting experimental tool and a potential new antidepressant agent.


