ImiquimodTLR-7 agonist,immunomodulator with antiviral and antitumor activity CAS# 99011-02-6 |
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Amantadine HCl
Catalog No.:BCC4465
CAS No.:665-66-7
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 99011-02-6 | SDF | Download SDF |
| PubChem ID | 57469 | Appearance | Powder |
| Formula | C14H16N4 | M.Wt | 240.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R 837 | ||
| Solubility | DMSO : 1.43 mg/mL (5.95 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
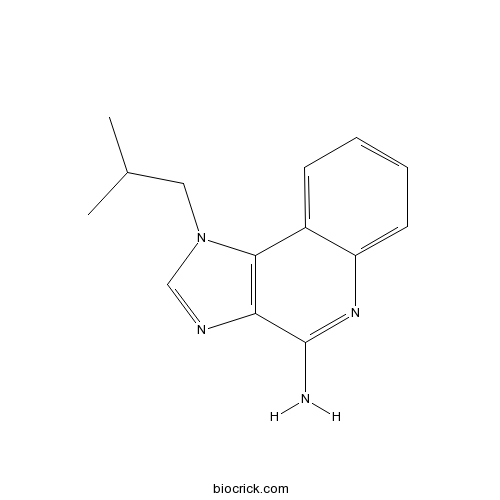
| Chemical Name | 1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine | ||
| SMILES | CC(C)CN1C=NC2=C1C3=CC=CC=C3N=C2N | ||
| Standard InChIKey | DOUYETYNHWVLEO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16N4/c1-9(2)7-18-8-16-12-13(18)10-5-3-4-6-11(10)17-14(12)15/h3-6,8-9H,7H2,1-2H3,(H2,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Immunomodulator that displays antiviral and antitumor activity. Acts as a Toll-like receptor 7 (TLR7) agonist; stimulates proinflammatory cytokine production and activates NF-κB. |

Imiquimod Dilution Calculator

Imiquimod Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1615 mL | 20.8073 mL | 41.6146 mL | 83.2293 mL | 104.0366 mL |
| 5 mM | 0.8323 mL | 4.1615 mL | 8.3229 mL | 16.6459 mL | 20.8073 mL |
| 10 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 50 mM | 0.0832 mL | 0.4161 mL | 0.8323 mL | 1.6646 mL | 2.0807 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Imiquimod activates toll-like receptor 7 (EC50 2.1 μM) on immune cells and also enables an OGF-mediated block of cell proliferation (IC50 2 μM) [1].
Toll-like receptor 7, also known as TLR7, is protein that in humans is encoded by the TLR7 gene. It is a member of the toll-like receptor (TLR) family. The TLR family plays an important role in pathogen recognition and activation of innate immunity. Imiquimod is a prescription medication that acts as an immune response modifier activating toll-like receptor 7. It is marketed by Meda AB, Graceway Pharmaceuticals, and iNova Pharmaceuticals under the trade names Aldara and Zyclara, and by Farmacoquímica Médica (FQM) in Brazil as Ixium.
In vitro: Results from a previous study demonstrate that imiquimod were capable of inducing interleukin-12 and interferon-g in mouse and human cell cultures. Imiquimod was also found to inhibit IL-4 and IL-5 production in mouse and human culture systems. These data suggested that imiquimod might have clinical utility in diseases where cellmediated immune responseswere important and in diseases associated with overexpression of IL-4 or IL-5, such as atopic disease. [2].
In vivo: The findings from animal study suggested that imiquimod could inhibit the airway inflammation of asthma animals by reducing GATA-3 mRNA and protein expression and increasing T-bet, STAT(6) mRNA and protein expression [3].
Clinical trial: Imiquimod is a patient-applied cream used to treat certain diseases of the skin, including skin cancers (basal cell carcinoma, Bowen's disease, superficial squamous cell carcinoma, some superficial malignant melanomas, and actinic keratosis) as well as genital warts (condylomata acuminata). However, Imiquimod is generally secondary to surgery, because surgery has a better chance to effectively treat at least some forms of skin cancer. Imiquimod has been tested for treatment of molluscum contagiosum. Two large randomized controlled trials, however, found no evidence of effectiveness of imiquimod in treating children with molluscum contagiosum, and concerning adverse effects were also noted. Imiquimod has also been tested for treatment of vulvar intraepithelial neoplasia, common warts that have proven difficult to treat, and vaginal intraepithelial neoplasia.
Reference:
[1] Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ. Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. Exp Biol Med (Maywood). 2008;233(8):968-79.
[2] Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, Reiter MJ, Vasilakos JP, Tomai MA. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191(1):10-9.
[3] Yin KS, Jin SX, Bian T, Wu QZ, Wang X, Yao X. The effects of imiquimod on an animal model of asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(7):509-17.
- Fentanyl citrate
Catalog No.:BCC6000
CAS No.:990-73-8
- 4-Hydroxybenzoic acid
Catalog No.:BCN4546
CAS No.:99-96-7
- 4'-Hydroxyacetophenone
Catalog No.:BCN4544
CAS No.:99-93-4
- 4-Isopropyltoluene
Catalog No.:BCC8282
CAS No.:99-87-6
- Methyl 4-hydroxybenzoate
Catalog No.:BCN4540
CAS No.:99-76-3
- Valproic acid
Catalog No.:BCC4260
CAS No.:99-66-1
- 3,4-Dihydroxybenzoic acid
Catalog No.:BCN4537
CAS No.:99-50-3
- 2-Methyl-5-Isopropenyl-2-Cyclohexenone
Catalog No.:BCC8279
CAS No.:99-49-0
- Chelidonic acid
Catalog No.:BCN6547
CAS No.:99-32-1
- Methyl gallate
Catalog No.:BCN3823
CAS No.:99-24-1
- Trehalose
Catalog No.:BCC9182
CAS No.:99-20-7
- Prunasin
Catalog No.:BCN4535
CAS No.:99-18-3
- Imiquimod hydrochloride
Catalog No.:BCC4196
CAS No.:99011-78-6
- [Ala107]-MBP (104-118)
Catalog No.:BCC5835
CAS No.:99026-77-4
- [Ala113]-MBP (104-118)
Catalog No.:BCC5836
CAS No.:99026-78-5
- Limonexic acid
Catalog No.:BCN4534
CAS No.:99026-99-0
- Kushenol I
Catalog No.:BCN2983
CAS No.:99119-69-4
- Kushenol E
Catalog No.:BCN3348
CAS No.:99119-72-9
- Kushenol C
Catalog No.:BCN3351
CAS No.:99119-73-0
- Yadanzioside I
Catalog No.:BCN6715
CAS No.:99132-95-3
- Yadanzioside L
Catalog No.:BCN6713
CAS No.:99132-97-5
- Dehydrobruceantinol
Catalog No.:BCN7621
CAS No.:99132-99-7
- 1,6,8-Trideoxyshanzhigenin
Catalog No.:BCN6909
CAS No.:99173-00-9
- Salannin
Catalog No.:BCN8052
CAS No.:992-20-1
Increased number of mast cells in the dermis in actinic keratosis lesions effectively treated with imiquimod.[Pubmed:28342266]
J Dermatol. 2017 Aug;44(8):944-949.
Actinic keratosis (AK) is a cutaneous cancer in situ which develops as a result of excessive exposure to ultraviolet (UV). Toll-like receptor (TLR)7 agonist Imiquimod is a topical immune response modifier and is effective for the treatment of non-melanoma skin cancers. Recently, the diagnostic role of the dermatoscope has been reported in the course of treatment of AK. In addition, mast cells are now considered to contribute to both the innate and adaptive immune systems in topical Imiquimod therapy. We assessed the effect of Imiquimod treatment by dermatoscopic and immunohistochemical findings in 14 patients with a total of 21 AK lesions. With the dermatoscope, though the mean erythema score was not significantly different between the cured lesions and the unresponsive lesions, the erythema/red pseudo-network ("strawberry") pattern was decreased significantly in the cured lesions. By immunohistochemistry, the number of Ki-67-positive proliferative cells in the epidermis was decreased and that of CD117-positive mast cells in the dermis was increased in the responding lesions. To the best of our knowledge, this is the first study demonstrating that the number of mast cells in the dermis was increased in AK lesions effectively treated with Imiquimod. Our present result suggests that mast cells may contribute an antitumor effect in human skin treated with topical Imiquimod.
A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer.[Pubmed:28341495]
Urol Oncol. 2017 Feb;35(2):39.e1-39.e7.
PURPOSE: Imiquimod is a toll-like receptor agonist with proven antitumor activity as a topical treatment for skin cancer. TMX-101 (Vesimune) is a novel liquid formulation of Imiquimod optimized for intravesical delivery. The agent demonstrated safety as an intravesical treatment for non-muscle-invasive bladder cancer in a phase 1 clinical trial. We report the results of a phase 2 prospective multicenter clinical trial assessing the safety and activity of TMX-101. MATERIALS AND METHODS: Patients with non-muscle-invasive bladder cancer containing carcinoma in situ were eligible for inclusion. Enrolled patients received 6 weekly intravesical administrations of 200mg/50ml TMX-101 0.4%. End points included rate of adverse events, changes in urinary cytokine levels following treatment, and clinical response at 6 weeks following final instillation, defined as negative posttreatment bladder biopsy and urine cytology results. RESULTS: A total of 12 patients were enrolled, with 10 available for efficacy analysis. Half of the patients (6/12) had received>/=2 prior induction courses of bacillus Calmette-Guerin. All patients received all 6 doses of TMX-101 per protocol. Overall, 75% of patients experienced treatment-related adverse events, only 1 of which was>grade 2 (urinary tract infection). Furthermore, 2 patients demonstrated a negative cytology and biopsy result at 6 weeks following treatment. Significant increases in urinary cytokines, including IL-6 and IL-18, were seen following treatment. CONCLUSION: In this phase 2 pilot study in patients with carcinoma in situ bladder cancer, intravesical TMX-101 was safe and well tolerated with common, mild genitourinary adverse effects. Clinical activity was suggested by the increase in posttreatment urinary cytokines. Complete responders were seen. Further investigation of the agent is warranted.
Vulvar condylomatosis after sex reassignment surgery in a male-to-female transsexual: Complete response to imiquimod cream.[Pubmed:28349118]
Gynecol Oncol Rep. 2017 Mar 18;20:75-77.
BACKGROUND: The number of patients seeking sex reassignment surgery is increasing. Approximately 1:30,000 adult males and 1: 100,000 adult females seek this procedure. Neovaginal-related disorders after sex reassignment surgery are increasingly more common. Vulvar condylomatosis is the clinical manifestation of HPV 6- and 11 infection in biological women. The same HPV-subtypes are associated with anogenital warts and penile intraepithelial neoplasia in biological men. We aim to present a case of vulvar condylomatosis after sex reassignment surgery in a male-to-female transsexual and its complete response to 5% Imiquimod cream. CASE: We describe a case of a 19-year-old female transexual who presented one year after male to female sex reassignment surgery by inverted penile skin vaginoplasty with condyloma accuminata of the vulva. The patient had a complete response to Imiquimod 5% cream 12 weeks after initiation of treatment. CONCLUSION: Gynecologists should be prepared to treat neovaginal-related disorders in male-to-female transsexuals.
The pharmacology of endosomal TLR agonists in viral disease.[Pubmed:18031247]
Biochem Soc Trans. 2007 Dec;35(Pt 6):1468-72.
The discovery of endosomal TLRs (Toll-like receptors) and their natural ligands has accelerated efforts to exploit them for therapeutic benefit. Importantly, this was preceded by clinical exploration of agents now known to be endosomal TLR agonists. Clinical effects in viral disease have been reported with agonists of TLR3, TLR7, TLR7/8 and TLR9, and the TLR7 agonist Imiquimod is marketed for topical use against warts, a papillomavirus disease. The observed pre-clinical and clinical profiles of agonists of each of these TLRs suggest induction of a multifaceted innate immune response, with biomarker signatures indicative of type 1 interferon induction. However, these agents differ in both their pharmaceutical characteristics and the cellular distribution of their target TLRs, suggesting that drugs directed to these targets will display differences in their overall pharmacological profiles.
The antitumoral mode of action of imiquimod and other imidazoquinolines.[Pubmed:17346155]
Curr Med Chem. 2007;14(6):681-7.
Imiquimod, the lead compound of the imidazoquinoline family of nucleoside analogues, has shown good efficacy against a variety of tumors of different origin. The mode of action of Imiquimod and related compounds, which we have begun to understand in some detail in recent years, is complex and interesting inasmuch as it appears to comprise several presumably mutually enhancing components. Predominant amongst its actions is the induction of pro-inflammatory cytokines through agonistic activity towards Toll-like receptor (TLR)-7 and TLR-8, and consecutively, activation of the central transcription factor NF-kappaB. This activity stimulates the production of pro-inflammatory cytokines, chemokines and other mediators resulting in activation of antigen-presenting cells and the mounting of a profound Th1-weighted antitumoral cellular immune response. In addition, there are a number of secondary effects on the molecular and cellular level that can be explained through the activation of NF-kappaB. The pro-inflammatory activity of Imiquimod appears to be augmented by suppression of a negative regulatory feedback mechanism which normally limits inflammatory responses. This is achieved independent of TLR-7 and TLR-8 through interference with adenosine receptor signaling pathways, particularly the A(2A) subtype, and receptor-independent reduction of adenylyl cyclase activity. Finally, at higher, albeit therapeutically relevant concentrations, Imiquimod exerts a pro-apoptotic activity against tumor cells. Induction of apoptosis by Imiquimod appears to be dependent on Bcl-2 proteins and involves caspase activation. The combination of multiple, presumably synergistic anti-tumoral functions by a single compound represents an interesting principle of pathogenesis-oriented, anti-neoplastic therapy.


