Imatinib hydrochlorideV-Abl/c-Kit/PDGFR inhibitor CAS# 862366-25-4 |
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 862366-25-4 | SDF | Download SDF |
| PubChem ID | 10437009 | Appearance | Powder |
| Formula | C29H32ClN7O | M.Wt | 530.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | 25℃: DMSO | ||
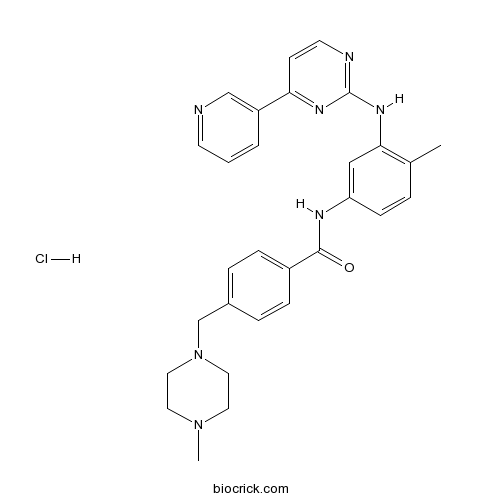
| Chemical Name | 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide;hydrochloride | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.Cl | ||
| Standard InChIKey | JWOCADZZAHSVDR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H31N7O.ClH/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Imatinib hydrochloride Dilution Calculator

Imatinib hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8866 mL | 9.4329 mL | 18.8658 mL | 37.7316 mL | 47.1645 mL |
| 5 mM | 0.3773 mL | 1.8866 mL | 3.7732 mL | 7.5463 mL | 9.4329 mL |
| 10 mM | 0.1887 mL | 0.9433 mL | 1.8866 mL | 3.7732 mL | 4.7164 mL |
| 50 mM | 0.0377 mL | 0.1887 mL | 0.3773 mL | 0.7546 mL | 0.9433 mL |
| 100 mM | 0.0189 mL | 0.0943 mL | 0.1887 mL | 0.3773 mL | 0.4716 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 100 nM (PDGFR) [1]; 100 nM (c-Kit) [2] Imatinib is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib is used to treat chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies. in vitro: In vitro assays for inhibition of a panel of tyrosine and serine/threonine protein kinases show that Imatinib inhibits the v-Abl tyrosine kinase and PDGFR potently with an IC50 of 0.6 and 0.1 μM, respectively [1]. Imatinib inhibits the SLF-dependent activation of wild-type c-kit kinase activity with a IC50 for these effects of approximately 0.1 μM, which is similar to the concentration required for inhibition of PDGFR [2]. Imatinib exhibits growth-inhibitory activity on the human bronchial carcinoid cell line NCI-H727 and the human pancreatic carcinoid cell line BON-1 with an IC50 of 32.4 and 32.8 μM, respectively [3]. in vivo: In the PS-ASODN group, tumor growth was inhibited by 59.437%, which was markedly higher than in the imatinib group (11.071%) and liposome negative control group [4]. Cohorts of mice were maintained on chow formulated with imatinib 0.5 mg/g or control chow for the duration of the experiment [5]. Toxicity: Imatinib is mainly indicated for chronic myeloid leukemia and gastrointestinal stromal tumors but is also prescribed by dermatologists for dermatofibrosarcoma protuberans, systemic sclerosis, and systemic mastocytosis, among other conditions. Most adverse effects are mild or moderate and therapy is generally well tolerated [6]. Clinical trial: Imatinib Mesylate And Mycophenolate Mofetil For Steroid-Refractory Sclerotic/Fibrotic cGVHD In Children. Phase 2
- RA VII
Catalog No.:BCN3512
CAS No.:86229-97-2
- Valeriotriate B
Catalog No.:BCN6751
CAS No.:862255-64-9
- Mirodenafil
Catalog No.:BCC5254
CAS No.:862189-95-5
- Nandrolone undecylate
Catalog No.:BCC9090
CAS No.:862-89-5
- Anamorelin hydrochloride
Catalog No.:BCC1364
CAS No.:861998-00-7
- 7-Methoxy-3,4-dihydro-1-naphthalenylacetonitrile
Catalog No.:BCC8781
CAS No.:861960-34-1
- 2''-O-Beta-L-Galorientin
Catalog No.:BCN3804
CAS No.:861691-37-4
- A-740003
Catalog No.:BCC1322
CAS No.:861393-28-4
- SKF 86466 hydrochloride
Catalog No.:BCC7795
CAS No.:86129-54-6
- Fmoc-D-Trp-OH
Catalog No.:BCC3559
CAS No.:86123-11-7
- Fmoc-D-Phe-OH
Catalog No.:BCC3537
CAS No.:86123-10-6
- 5'-Fluoroindirubinoxime
Catalog No.:BCC6104
CAS No.:861214-33-7
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- IKK-3 Inhibitor
Catalog No.:BCC1643
CAS No.:862812-98-4
- Salvianan
Catalog No.:BCN3545
CAS No.:862832-46-0
- Ganoderic acid TR
Catalog No.:BCN3207
CAS No.:862893-75-2
- Isoiridogermanal
Catalog No.:BCN7613
CAS No.:86293-25-6
- 10-Hydroxycanthin-6-one
Catalog No.:BCN3906
CAS No.:86293-41-6
- alpha-Amyrin acetate
Catalog No.:BCN4410
CAS No.:863-76-3
- Azilsartan Medoxomil
Catalog No.:BCC5021
CAS No.:863031-21-4
- Azilsartan medoxomil monopotassium
Catalog No.:BCC4089
CAS No.:863031-24-7
- Impurity C of Calcitriol
Catalog No.:BCC5384
CAS No.:86307-44-0
- Dasatinib monohydrate
Catalog No.:BCN2177
CAS No.:863127-77-9
- 8-epi-Chlorajapolide F
Catalog No.:BCN6426
CAS No.:863301-69-3
Advanced chordoma treated by first-line molecular targeted therapies: Outcomes and prognostic factors. A retrospective study of the French Sarcoma Group (GSF/GETO) and the Association des Neuro-Oncologues d'Expression Francaise (ANOCEF).[Pubmed:28478340]
Eur J Cancer. 2017 Jul;79:119-128.
BACKGROUND: To assess the role of first-line Molecular Targeted Therapies (MTTs) in Advanced chordoma (AC) patients. METHODS: Retrospective study of 80 patients treated between January 2004 and December 2015 at 15 major French Sarcoma or Neurooncology Centres. RESULTS: The sex ratio M/F was 46/34. The median age was 59 (6-86) years. The primary sites were the sacrum (50, 62.5%), mobile spine (12, 15.0%), and skull base (18, 22.5%). Metastases were present in 28 patients (36.0%). The first line of MTTs consisted of imatinib (62, 77.5%), sorafenib (11, 13.7%), erlotinib (5, 6.3%), sunitinib (1, 1.2%) and temsirolimus (1, 1.2%). The reported responses were: partial response (5, 6.3%), stable disease (58, 72.5%), or progressive disease (10, 12.5%). Symptomatic improvement was seen in 28/66 assessable patients (42.4%) and was associated with an objective response occurrence (p = 0.005), imatinib (p = 0.020) or erlotinib use (p = 0.028). The median progression-free survival (PFS) was 9.4 degrees months (95% CI, [6.8-16.1]). Two independent factors of poor prognosis for PFS were identified: a skull-based primary location (HR = 2.5, p = 0.019), and the interval between diagnosis and MTT of <52months (HR = 2.8, p < 0.001). The median overall survival (OS) was 4.4 degrees years (95% CI, [3.8-5.6]). Four independent factors of poor prognosis for OS were identified: the presence of liver metastases (HR = 13.2, p < 0.001), pain requiring opioids (HR = 2.9, p = 0.012), skull-based primary location (HR = 19.7, p < 0.001), and prior radiotherapy (photon alone) (HR = 2.5, p = 0.024). The PFS and OS did not significantly differ between the MTT. CONCLUSIONS: The prognostic factors identified require validation in an independent database but are potently useful to guide treatment decisions and design further clinical trials.
Hiding inside? Intracellular expression of non-glycosylated c-kit protein in cardiac progenitor cells.[Pubmed:27161312]
Stem Cell Res. 2016 May;16(3):795-806.
Cardiac progenitor cells including c-kit(+) cells and cardiosphere-derived cells (CDCs) play important roles in cardiac repair and regeneration. CDCs were reported to contain only small subpopulations of c-kit(+) cells and recent publications suggested that depletion of the c-kit(+) subpopulation of cells has no effect on regenerative properties of CDCs. However, our current study showed that the vast majority of CDCs from murine heart actually express c-kit, albeit, in an intracellular and non-glycosylated form. Immunostaining and flow cytometry showed that the fluorescent signal indicative of c-kit immunostaining significantly increased when cell membranes were permeabilized. Western blots further demonstrated that glycosylation of c-kit was increased during endothelial differentiation in a time dependent manner. Glycosylation inhibition by 1-deoxymannojirimycin hydrochloride (1-DMM) blocked c-kit glycosylation and reduced expression of endothelial cell markers such as Flk-1 and CD31 during differentiation. Pretreatment of these cells with a c-kit kinase inhibitor (imatinib mesylate) also attenuated Flk-1 and CD31 expression. These results suggest that c-kit glycosylation and its kinase activity are likely needed for these cells to differentiate into an endothelial lineage. In vivo, we found that intracellular c-kit expressing cells are located in the wall of cardiac blood vessels in mice subjected to myocardial infarction. In summary, our work demonstrated for the first time that c-kit is not only expressed in CDCs but may also directly participate in CDC differentiation into an endothelial lineage.
Ductular reaction correlates with fibrogenesis but does not contribute to liver regeneration in experimental fibrosis models.[Pubmed:28445529]
PLoS One. 2017 Apr 26;12(4):e0176518.
BACKGROUND AND AIMS: Ductular reaction is a standard component of fibrotic liver tissue but its function is largely unknown. It is supposed to interact with the matrix producing myofibroblasts and compensate the declining regenerative capacity of hepatocytes. The relationship between the extent of fibrosis-ductular reaction, proliferative activity of hepatocytes and ductular reaction were studied sequentially in experimental hepatic fibrosis models. METHODS: Liver fibrosis/cirrhosis was induced in wild type and TGFbeta overproducing transgenic mice by carbon tetrachloride and thioacetamide administration. The effect of thioacetamide was modulated by treatment with imatinib and erlotinib. The extent of ductular reaction and fibrosis was measured by morphometry following cytokeratin 19 immunofluorescent labeling and Picro Sirius staining respectively. The proliferative activity of hepatocytes and ductular reaction was evaluated by BrdU incorporation. The temporal distribution of the parameters was followed and compared within and between different experimental groups. RESULTS: There was a strong significant correlation between the extent of fibrosis and ductular reaction in each experimental group. Although imatinib and erlotinib temporarily decreased fibrosis this effect later disappeared. We could not observe negative correlation between the proliferation of hepatocytes and ductular reaction in any of the investigated models. CONCLUSIONS: The stringent connection between ductular reaction and fibrosis, which cannot be influenced by any of our treatment regimens, suggests that there is a close mutual interaction between them instead of a unidirectional causal relationship. Our results confirm a close connection between DR and fibrogenesis. However, since the two parameters changed together we could not establish a causal relationship and were unable to reveal which was the primary event. The lack of inverse correlation between the proliferation of hepatocytes and ductular reaction questions that ductular reaction can compensate for the failing regenerative activity of hepatocytes. No evidences support the persistent antifibrotic property of imatinib or erlotinib.
Investigation of imatinib loaded surface decorated biodegradable nanocarriers against glioblastoma cell lines: Intracellular uptake and cytotoxicity studies.[Pubmed:27154254]
Int J Pharm. 2016 Jun 30;507(1-2):61-71.
Overexpression of P-glycoprotein (P-gp) efflux transporter in glioma cells thwarts the build-up of therapeutic concentration of drugs usually resulting into poor therapeutic outcome. To surmount aforesaid challenge, Imatinib (IMM) loaded Poly-lactide-co-glycolic acid nanoparticles (IMM-PLGA-NPs) were developed and optimized by Box Behnken Design as a new treatment stratagem in malignant glioma. Optimized NPs were functionalized with Pluronic((R)) P84, P-gp inhibitor (IMM-PLGA-P84-NPs) which showed size, PDI, zeta potential, drug loading, 182.63+/-13.56nm, 0.196+/-0.021, -15.2+/-1.49mV, 40.63+/-2.04mug/mg, respectively. Intracellular uptake study conducted on A172, U251MG and C6 glioma cells demonstrated significantly high uptake of IMM through NPs when compared with IMM solution (IMM-S), p<0.001. IMM-PLGA-P84-NPs showed better uptake in P-gp expressing cell line (U251MG and C6) while uncoated NPs showed higher uptake in non-P-gp expressing cell line (A-172). Cytotoxicity studies demonstrated significantly low IC50 for both IMM-PLGA-NPs and IMM-PLGA-P84-NPs when compared with IC50 of IMM-S. IMM-PLGA-P84-NPs showed a significantly low IC50 against P-gp overexpressing cell lines when compared with IC50 of IMM-PLGA-NPs. In contrary, IMM-PLGA-NPs showed lower IC50 against non P-gp expressing cell line. This study demonstrated the feasibility of targeting surface decorated NPs to multidrug resistant gliomas. However, to address its clinical utility extensive in vivo studies are required.
Efficacy of erlotinib and imatinib in a patient with a rectal gastrointestinal stromal tumor and synchronous pulmonary adenocarcinoma: A case report.[Pubmed:27040071]
J Med Invest. 2016;63(1-2):144-8.
The synchronous existence of lung cancer and gastrointestinal stromal tumors (GIST) is considered to be extremely rare. To the best of our knowledge, this is the first report about the treatment of lung cancer and GIST with two kinds of molecular targeting drugs. An 83-year-old woman with a rectal GIST, which carried a c-kit mutation, and pulmonary adenocarcinoma, which exhibited an epidermal growth factor receptor (EGFR) mutation, was treated alternately with imatinib and erlotinib. Good control over both diseases was achieved for two years. The present case is not only of interest due to the rare co-occurrence of GIST and lung cancer, but also because it involved two tumors carrying different gene mutations, and both tumors were brought under control using different molecular targeting drugs.


