GenisteinAR agonist CAS# 446-72-0 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
Number of papers citing our products

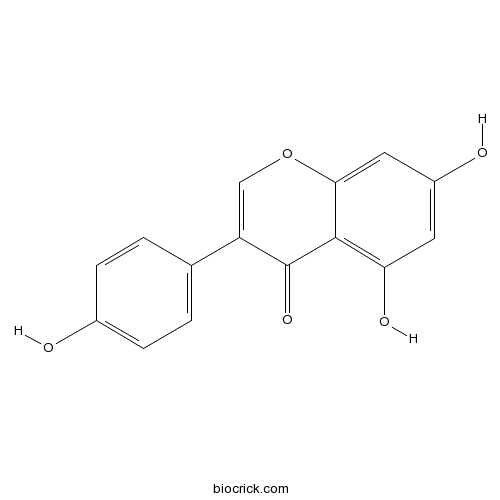
Chemical structure

3D structure
| Cas No. | 446-72-0 | SDF | Download SDF |
| PubChem ID | 5280961 | Appearance | Slightly yellowish powder |
| Formula | C15H10O5 | M.Wt | 270.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | NPI 031L | ||
| Solubility | DMSO : ≥ 100 mg/mL (370.04 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O | ||
| Standard InChIKey | TZBJGXHYKVUXJN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Genistein, a phytoestrogen found in soy products, is a highly specific inhibitor of protein tyrosine kinase (PTK) which blocks the mitogenic effect mediated by EGF on NIH-3T3 cells with IC50 of 12μM or by insulin with IC50 of 19 μM. Genistein has neuroprotective, antitumor effects, it modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats. |
| Targets | TGF-β/Smad | NF-kB | p38MAPK | ERK | Bcl-2/Bax | NOS | COX | NO | PGE | IkB | cAMP | p65 | Beta Amyloid | Estrogen receptor | IL Receptor | IKK | Progestogen receptor |
| In vitro | Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: partial mediation by the transcription factor NFAT1.[Pubmed: 24243709]Mol Carcinog. 2015 Apr;54(4):301-11.To investigate the effects and mechanism of Genistein on hepatocellular carcinoma. Genistein inhibits the replication of avian leucosis virus subgroup J in DF-1 cells.[Pubmed: 25197039]Virus Res. 2014 Nov 4;192:114-20.To investigate the antiviral effects of Genistein on the replication of avian leukosis virus subgroup J (ALV-J) in DF-1 cells, the cells were treated with Genistein at different time points and the antiviral effects were examined by using a variety of assays. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells.[Pubmed: 25202045]Anticancer Res. 2014 Sep;34(9):4685-92.Although 5-fluorouracil (5-FU)-based combination chemotherapy (i.e. FOLFIRINOX) has demonstrated effectiveness against pancreatic cancer, novel therapeutic strategies must be developed to increase the therapeutic window of these cytotoxic agents. Genistein is a soy-derived isoflavone with pleiotropic biological effects that can enhance the antitumor effect of chemotherapeutic agents.
Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity.[Pubmed: 15207258 ]Neurobiol Dis. 2004 Jun;16(1):21-8.Estrogen is beneficial to patients with Alzheimer's disease (AD) but has a limited clinical use due to its proliferative and oncogenic effects on non-neuronal cells responsive to estrogen. |
| Kinase Assay | Molecular effects of genistein on estrogen receptor mediated pathways.[Pubmed: 8625449]Genistein inhibits phorbol ester-induced NF-κB transcriptional activity and COX-2 expression by blocking the phosphorylation of p65/RelA in human mammary epithelial cells.[Pubmed: 24742714]Mutat Res. 2014 Oct;768:74-83.Genistein, an isoflavone present in soy products, has chemopreventive effects on mammary carcinogenesis. Carcinogenesis. 1996 Feb;17(2):271-5.Genistein, a component of soy products, may play a role in the prevention of breast and prostate cancer. However, little is known about the molecular mechanisms involved. |
| Animal Research | Genistein modulates the expression of NF-κB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats.[Pubmed: 25620059]Toxicol Appl Pharmacol. 2015 Mar 1;283(2):139-46.Genistein is an isoflavanoid abundantly found in soy. It has been found to play an important role in the prevention of various chronic diseases including cancer. In this study, we evaluated potential therapeutic properties of Genistein against d-Galactosamine (d-GalN) induced inflammation and hepatotoxicity in male Wistar rats. |

Genistein Dilution Calculator

Genistein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.701 mL | 18.5048 mL | 37.0096 mL | 74.0192 mL | 92.5241 mL |
| 5 mM | 0.7402 mL | 3.701 mL | 7.4019 mL | 14.8038 mL | 18.5048 mL |
| 10 mM | 0.3701 mL | 1.8505 mL | 3.701 mL | 7.4019 mL | 9.2524 mL |
| 50 mM | 0.074 mL | 0.3701 mL | 0.7402 mL | 1.4804 mL | 1.8505 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.3701 mL | 0.7402 mL | 0.9252 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Phytoestrogen with a wide range of biological actions. Inhibits protein tyrosine kinases including epidermal growth factor receptor kinase. Also binds to PPARγ and estrogen receptors and acts as an agonist at GPR30. Also available as part of the MAP
- Homoeriodictyol
Catalog No.:BCN6804
CAS No.:446-71-9
- BMS CCR2 22
Catalog No.:BCC7572
CAS No.:445479-97-0
- SGS 518 oxalate
Catalog No.:BCC7750
CAS No.:445441-27-0
- BMS-345541(free base)
Catalog No.:BCC5374
CAS No.:445430-58-0
- ZM 449829
Catalog No.:BCC2444
CAS No.:4452-06-6
- 1,4:3,6-Dianhydro-α-D-glucopyranose
Catalog No.:BCC8420
CAS No.:4451-30-3
- EBPC
Catalog No.:BCC6677
CAS No.:4450-98-0
- TC-O 9311
Catalog No.:BCC7900
CAS No.:444932-31-4
- AM1241
Catalog No.:BCC2551
CAS No.:444912-48-5
- Warangalone
Catalog No.:BCN4788
CAS No.:4449-55-2
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- CMX001
Catalog No.:BCC4106
CAS No.:444805-28-1
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- 2,4,5-Trimethoxybenzaldehyde
Catalog No.:BCN5498
CAS No.:4460-86-0
- YM 230888
Catalog No.:BCC5956
CAS No.:446257-23-4
- 4-(4-(5-(Aminomethyl)-2-oxooxazolidin-3-yl)phenyl)morpholin-3-one
Catalog No.:BCC8646
CAS No.:446292-10-0
- RepSox
Catalog No.:BCC1887
CAS No.:446859-33-2
- Angiotensin II human
Catalog No.:BCC4087
CAS No.:4474-91-3
- RLLFT-NH2
Catalog No.:BCC3954
CAS No.:447408-68-6
- Ruixianglangdusu B
Catalog No.:BCN6869
CAS No.:447454-49-1
- Sulforaphane
Catalog No.:BCN2349
CAS No.:4478-93-7
- Betulonic acid
Catalog No.:BCN5500
CAS No.:4481-62-3
- NS 1643
Catalog No.:BCC7552
CAS No.:448895-37-2
- WY 45233 succinate
Catalog No.:BCC6125
CAS No.:448904-47-0
Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells.[Pubmed:25202045]
Anticancer Res. 2014 Sep;34(9):4685-92.
BACKGROUND: Although 5-fluorouracil (5-FU)-based combination chemotherapy (i.e. FOLFIRINOX) has demonstrated effectiveness against pancreatic cancer, novel therapeutic strategies must be developed to increase the therapeutic window of these cytotoxic agents. Genistein is a soy-derived isoflavone with pleiotropic biological effects that can enhance the antitumor effect of chemotherapeutic agents. MATERIALS AND METHODS: To understand how Genistein potentiates the antitumor effects of 5-FU, we examined apoptosis and autophagy in MIA PaCa-2 human pancreatic cancer cells and their derived xenografts. Apoptosis was evaluated using DNA fragmentation assays, and western blots of poly(ADP ribose)polymerase and caspase-3. Meanwhile, autophagy was evaluated using western blots of microtubule-associated protein light chain 3 (LC3)-I/II, fluorescent microscopy observation of green fluorescent protein-LC3B puncta formation, and acidic vesicular organelle formation using acridine orange staining. Tumors from animal treatment studies were examined for apoptosis and autophagy using the TdT-mediated dUTP nick-end labeling assay and immunohistochemical staining of LC3B, respectively. RESULTS: We observed that Genistein increased 5-FU-induced cell death through increased apoptosis, as well as autophagy. The increased autophagy was accompanied by decreased B-cell lymphoma 2 (Bcl2) and increased beclin-1 protein levels. Animal treatment studies supported these observations. The combination of 5-FU and Genistein significantly reduced final xenograft tumor volume when compared to 5-FU-alone by inducing apoptosis as well as autophagy. CONCLUSION: Genistein can potentiate the antitumor effect of 5-FU by inducing apoptotic as well as autophagic cell death. These results demonstrate the potential of Genistein as an adjuvant therapeutic agent against pancreatic cancer.
Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity.[Pubmed:15207258]
Neurobiol Dis. 2004 Jun;16(1):21-8.
Estrogen is beneficial to patients with Alzheimer's disease (AD) but has a limited clinical use due to its proliferative and oncogenic effects on non-neuronal cells responsive to estrogen. In an attempt to find an estrogen substitute that retains the beneficial effects of estrogen with minimal side effects, we compared the neuroprotective and proliferative effects of Genistein, a selective estrogen receptor (ER) beta-agonist, with those of estrogen. Genistein and 17beta-estradiol showed comparable levels of protection against Abeta-induced deaths of cultured SH-SY5Y human neuroblastoma cells, which were blocked by an estrogen receptor antagonist, ICI 182,780. On the other hand, 17beta-estradiol, but not Genistein, induced proliferation of uterine endometrial cells. Our results suggest that Genistein is a potential alternative to estrogen in the treatment of Alzheimer's disease.
Genistein inhibits the replication of avian leucosis virus subgroup J in DF-1 cells.[Pubmed:25197039]
Virus Res. 2014 Nov 4;192:114-20.
To investigate the antiviral effects of Genistein on the replication of avian leukosis virus subgroup J (ALV-J) in DF-1 cells, the cells were treated with Genistein at different time points and the antiviral effects were examined by using a variety of assays. We determined that Genistein strongly inhibited viral gene expression and decreased the viral protein level in the cell supernatant and the cytoplasm without alerting virus receptor expression and viral attachment. We also observed that Genistein was not found to interfere with virus entry, but significantly inhibited both viral gene transcriptions at 24h post infection and virus release, which indicate that Genistein exerts its inhibitory effects on the late phase of ALV-J replicative cycle. These results demonstrate that Genistein effectively block ALV-J replication by inhibiting virus transcription and release in DF-1 cells, which may be useful for therapeutic drug design.
Molecular effects of genistein on estrogen receptor mediated pathways.[Pubmed:8625449]
Carcinogenesis. 1996 Feb;17(2):271-5.
Genistein, a component of soy products, may play a role in the prevention of breast and prostate cancer. However, little is known about the molecular mechanisms involved. In the present study, we examined the effects of Genistein on the estrogen receptor positive human breast cancer cell line MCF-7. We observed that Genistein stimulated estrogen-responsive pS2 mRNA expression at concentrations as low as 10(-8) M and these effects can be inhibited by tamoxifen. We also showed that Genistein competed with [3H]estradiol binding to the estrogen receptor with 50% inhibition at 5 x 10(-7) M. Thus, the estrogenic effect of Genistein would appear to be a result of an interaction with the estrogen receptor. The effect of Genistein on growth of MCF-7 cells was also examined. Genistein produced a concentration-dependent effect on the growth of MCF-7 cells. At lower concentrations (10(-8)-10(-6) M) Genistein stimulated growth, but at higher concentrations (> 10(-5) M) Genistein inhibited growth. The effects of Genistein on growth at lower concentrations appeared to be via the estrogen receptor pathway, while the effects at higher concentrations were independent of the estrogen receptor. We also found that Genistein, though estrogenic, can interfere with the effects of estradiol. In addition, prolonged exposure to Genistein resulted in a decrease in estrogen receptor mRNA level as well as a decreased response to stimulation by estradiol.
Genistein inhibits hepatocellular carcinoma cell migration by reversing the epithelial-mesenchymal transition: partial mediation by the transcription factor NFAT1.[Pubmed:24243709]
Mol Carcinog. 2015 Apr;54(4):301-11.
To investigate the effects and mechanism of Genistein on hepatocellular carcinoma. Cell counting kit-8 assays showed that Genistein at 3, 6, and 9 microM had no significant cytotoxic effects on HepG2, SMMC-7721, and Bel-7402 cells. Cell scratch and Transwell assays identified that Genistein inhibited migration of three cell lines. In three cell lines, Genistein enhanced E-cadherin and alpha-catenin, but reduced N-cadherin and Vimentin at both mRNA and protein levels in a dose-dependent manner. Simultaneously, treatment with Genistein suppressed epithelial-mesenchymal transition (EMT) induced by TGF-beta. In HepG2 cells, Genistein reduced mRNA, and protein expressions of nuclear factor of activated T cells 1 (NFAT1), Abca3, Autotaxin, CD154, and Cox-2. Phorbol 12-myristate 13-acetate (PMA) and ionomycin enhanced activity of NFAT1, reduced E-cadherin and alpha-catenin protein levels, and increased protein levels of N-cadherin and Vimentin. Transwell demonstrated that PMA and ionomycin reversed the migration inhibitory effects of Genistein on HepG2 cells. In vivo, Genistein inhibited the intrahepatic metastasis by reversing the EMT, which was correlated with reduced NFAT1 . Genistein inhibited hepatocellular carcinoma cell migration by reversing the EMT, which was partly mediated by NFAT1. The fact that EMT can be reversed by Genistein may shed light on the possible mechanisms for its role in liver cancer therapy.
Genistein modulates the expression of NF-kappaB and MAPK (p-38 and ERK1/2), thereby attenuating d-Galactosamine induced fulminant hepatic failure in Wistar rats.[Pubmed:25620059]
Toxicol Appl Pharmacol. 2015 Mar 1;283(2):139-46.
Genistein is an isoflavanoid abundantly found in soy. It has been found to play an important role in the prevention of various chronic diseases including cancer. In this study, we evaluated potential therapeutic properties of Genistein against d-Galactosamine (d-GalN) induced inflammation and hepatotoxicity in male Wistar rats. Fulminant hepatic failure (FHF) was induced in rats by intraperitoneal injection of d-GalN (700mg/kgBW). Genistein (5mg/kgBW/day) was given as pre-treatment for 30days via intra-gastric route followed by d-GalN (700mg/kgBW) injection. The hepatoprotective and curative effects of Genistein were evident from a significant decrease in the serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels as well as prevention of histological damage by pre-treatment of Genistein. Genistein pre-treatment significantly inhibited the increased protein levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), thereby reducing nitric oxide (NO) and prostaglandin-E2 (PGE) levels, respectively. In addition Genistein significantly suppressed the production of d-GalN-induced proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-1beta. These inhibitory effects were associated with the suppression of nuclear factor-kappa B (NF-kB) activation, IKKalpha/beta and Mitogen activated protein kinase (MAPK) phosphorylation by Genistein in d-GalN-treated animals. In conclusion, our results suggest that Genistein may serve as a potential supplement in the prevention of hepatic and inflammatory diseases. Furthermore Genistein is able to maintain the redox potential and strengthens the antioxidant defense system of a cell.
Genistein inhibits phorbol ester-induced NF-kappaB transcriptional activity and COX-2 expression by blocking the phosphorylation of p65/RelA in human mammary epithelial cells.[Pubmed:24742714]
Mutat Res. 2014 Oct;768:74-83.
Genistein, an isoflavone present in soy products, has chemopreventive effects on mammary carcinogenesis. In the present study, we have investigated the effects of Genistein on phorbol ester-induced expression of cyclooxygenase-2 (COX-2) that plays an important role in the pathophysiology of inflammation-associated carcinogenesis. Pretreatment of cultured human breast epithelial (MCF10A) cells with Genistein reduced COX-2 expression induced by 12-O-tetradecanoylphorbol-13-acetate (TPA). There are multiple lines of evidence supporting that the induction of COX-2 is regulated by the eukaryotic transcription factor NF-kappaB. Genistein failed to inhibit TPA-induced nuclear translocation and DNA binding of NF-kappaB as well as degradation of IkappaB. However, Genistein abrogated the TPA-induced transcriptional activity of NF-kappaB as determined by the luciferase reporter gene assay. Genistein inhibited phosphorylation of the p65 subunit of NF-kappaB and its interaction with cAMP regulatory element-binding protein-binding protein (CBP)/p300 and TATA-binding protein (TBP). TPA-induced NF-kappaB phosphorylation was abolished by pharmacological inhibition of extracellular signal-regulated kinase (ERK). Likewise, pharmacologic inhibition or dominant negative mutation of ERK suppressed phosphorylation of p65. The above findings, taken together, suggest that Genistein inhibits TPA-induced COX-2 expression in MCF10A cells by blocking ERK-mediated phosphorylation of p65 and its subsequent interaction with CBP and TBP.
Peroxisome proliferator-activated receptor gamma (PPARgamma ) as a molecular target for the soy phytoestrogen genistein.[Pubmed:12421816]
J Biol Chem. 2003 Jan 10;278(2):962-7.
The principal soy phytoestrogen Genistein has an array of biological actions. It binds to estrogen receptor (ER) alpha and beta and has ER-mediated estrogenic effects. In addition, it has antiestrogenic effects as well as non-ER-mediated effects such as inhibition of tyrosine kinase. Because of its complex biological actions, the molecular mechanisms of action of Genistein are poorly understood. Here we show that Genistein dose-dependently increases estrogenic transcriptional activity in mesenchymal progenitor cells, but its biological effects on osteogenesis and adipogenesis are different. At low concentrations (< or =1 microm), Genistein acts as estrogen, stimulating osteogenesis and inhibiting adipogenesis. At high concentrations (>1 microm), however, Genistein acts as a ligand of PPARgamma, leading to up-regulation of adipogenesis and down-regulation of osteogenesis. Transfection experiments show that activation of PPARgamma by Genistein at the micromolar concentrations down-regulates its estrogenic transcriptional activity, while activation of ERalpha or ERbeta by Genistein down-regulates PPARgamma transcriptional activity. Genistein concurrently activates two different transcriptional factors, ERs and PPARgamma, which have opposite effects on osteogenesis or adipogenesis. As a result, the balance between activated ERs and PPARgamma determines the biological effects of Genistein on osteogenesis and adipogenesis. Our findings may explain distinct effects of Genistein in different tissues.
Prevention of anti-CD3 monoclonal antibody-induced thymic apoptosis by protein tyrosine kinase inhibitors.[Pubmed:7523495]
J Immunol. 1994 Oct 15;153(8):3457-65.
The thymus gland is crucial for the formation of thymocytes of diverse TCR specificity. Recent studies have demonstrated that deletion (negative selection) of autoreactive thymocytes occurs through the process of apoptosis in which TCR activates cell death by DNA fragmentation. In addition, in vitro stimulation of thymocytes with anti-CD3 mAb, calcium ionophore, or glucocorticoids results in DNA fragmentation followed by cell death. The availability of various substances capable of inhibiting activation-induced programmed cell death of thymocytes may be used as a tool to help identify several important events occurring during the process of apoptosis. We investigated the effect of protein tyrosine kinase (PTK) inhibitors, herbimycin A and Genistein, on thymocyte apoptosis induced by stimulation of anti-CD3 mAb or glucocorticoid. Anti-CD3 mAb stimulation resulted in removal of CD4+CD8+ thymocytes by DNA fragmentation. However, in PTK inhibitor-pretreated thymocytes, there was a minimal deletion of double positive thymocytes. In contrast, PTK inhibitors did not prevent glucocorticoid-induced thymic apoptosis. Our results suggest that anti-CD3 mAb-induced thymic apoptosis depends on PTK activation via TCR, and that glucocorticoid-induced thymic apoptosis is PTK-independent.
Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity.[Pubmed:2153378]
Biochem Pharmacol. 1990 Jan 1;39(1):187-93.
Genistein has been shown to inhibit specifically in vitro the epidermal growth factor (EGF)-receptor tyrosine protein kinase activity (Akiyama et al., J Biol Chem 262: 5592-5597, 1987). When the effects of Genistein on NIH-3T3 cells were studied, a cytostatic effect was observed at low concentration (less than 40 microM) and a cytotoxic effect at higher concentration (greater than 40 microM). Genistein was able to block the mitogenic effect mediated by EGF on NIH-3T3 cells (IC50 = 12 microM) or by insulin (IC50 = 19 microM). Genistein was also able to block the mitogenic effect mediated by thrombin (IC50 = 20 microM) although the thrombin receptor does not involve a protein tyrosine kinase activity. Genistein at cytostatic concentration (less than 40 microM) did not prevent the induction of c-myc mRNA synthesis caused by the activation of EGF receptor by EGF. Therefore the cytostatic effect of Genistein on NIH-3T3 cells did not appear to be mediated by EGF receptor tyrosine kinase inhibition. This hypothesis was also supported by the absence of effect of Genistein on the EGF-stimulated phosphorylation of several proteins and particularly of a cytosolic 80 kD protein. The stimulation of S6 kinase activity of cells treated by EGF was prevented by Genistein. The stimulation by EGF of in situ S6 phosphorylation was also prevented by Genistein. In addition, S6 kinase extracted from cells treated by EGF was inhibited by Genistein. These effects occur at similar doses and maximal inhibition of S6 kinase was obtained at about 15 microM.
Genistein, a specific inhibitor of tyrosine-specific protein kinases.[Pubmed:3106339]
J Biol Chem. 1987 Apr 25;262(12):5592-5.
Tyrosine-specific protein kinase activity of the epidermal growth factor (EGF) receptor, pp60v-src and pp110gag-fes was inhibited in vitro by an isoflavone Genistein. The inhibition was competitive with respect to ATP and noncompetitive to a phosphate acceptor, histone H2B. By contrast, Genistein scarcely inhibited the enzyme activities of serine- and threonine-specific protein kinases such as cAMP-dependent protein kinase, phosphorylase kinase, and the Ca2+/phospholipid-dependent enzyme protein kinase C. When the effect of Genistein on the phosphorylation of the EGF receptor was examined in cultured A431 cells, EGF-stimulated serine, threonine, and tyrosine phosphorylation was decreased. Phosphoamino acid analysis of total cell proteins revealed that Genistein inhibited the EGF-stimulated increase in phosphotyrosine level in A431 cells.


