GW9662PPARγ antagonist CAS# 22978-25-2 |
- Pioglitazone HCl
Catalog No.:BCC2278
CAS No.:112529-15-4
- Rosiglitazone
Catalog No.:BCC2264
CAS No.:122320-73-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- T0070907
Catalog No.:BCC2261
CAS No.:313516-66-4
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 22978-25-2 | SDF | Download SDF |
| PubChem ID | 644213 | Appearance | Powder |
| Formula | C13H9ClN2O3 | M.Wt | 276.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (361.43 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
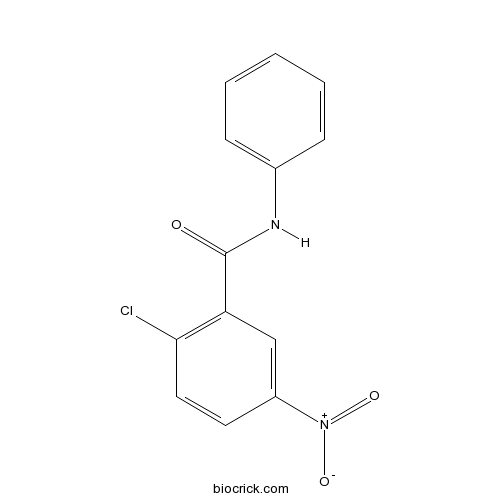
| Chemical Name | 2-chloro-5-nitro-N-phenylbenzamide | ||
| SMILES | C1=CC=C(C=C1)NC(=O)C2=C(C=CC(=C2)[N+](=O)[O-])Cl | ||
| Standard InChIKey | DNTSIBUQMRRYIU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H9ClN2O3/c14-12-7-6-10(16(18)19)8-11(12)13(17)15-9-4-2-1-3-5-9/h1-8H,(H,15,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective PPARγ antagonist (IC50 values are 3.3, 32 and 2000 nM for PPARγ, PPARα and PPARδ respectively). Blocks the inhibition of osteoclast formation induced by IL-4 in the low micromolar range (1-2 μM), therefore is more potent than BADGE. Anticancer, inhibits growth of human mammary tumor cell lines. |

GW9662 Dilution Calculator

GW9662 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6143 mL | 18.0714 mL | 36.1428 mL | 72.2857 mL | 90.3571 mL |
| 5 mM | 0.7229 mL | 3.6143 mL | 7.2286 mL | 14.4571 mL | 18.0714 mL |
| 10 mM | 0.3614 mL | 1.8071 mL | 3.6143 mL | 7.2286 mL | 9.0357 mL |
| 50 mM | 0.0723 mL | 0.3614 mL | 0.7229 mL | 1.4457 mL | 1.8071 mL |
| 100 mM | 0.0361 mL | 0.1807 mL | 0.3614 mL | 0.7229 mL | 0.9036 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW9662 is a potent antagonist of PPARγ with IC50 value of 3.3nM [1].
PPARγ belongs to the nuclear receptor superfamily, the inhibition of it is a strategy for treatment of several cancers, including breast. GW9662 acts as a potent, irreversible and selective PPARγ antagonist in both cell-free and cell-based assays. It modifies a cysteine residue in the ligand-binding site of PPARγ. In three breast cell lines including MCF7, MDA-MB-231 and MDA-MB-468, GW9662 suppresses the cell viability with IC50 values ranging from 20-30μM. In addition, GW9662 is reported to abolish the protection of LPS in a model of renal ischemia/reperfusion (I/R). In this model, the renal/glomerular dysfunction, tubular dysfunction and reperfusion injury caused by I/R can be significantly attenuated by LPS, and administration of GW9662 reverses this [2, 3].
References:
[1] Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010 Jun;41(2-3):180-6.
[2] Seargent JM, Yates EA, Gill JH. GW9662, a potent antagonist of PPARgamma, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation. Br J Pharmacol. 2004 Dec;143(8):933-7.
[3] Collino M, Patel NS, Lawrence KM, Collin M, Latchman DS, Yaqoob MM, Thiemermann C. The selective PPARgamma antagonist GW9662 reverses the protection of LPS in a model of renal ischemia-reperfusion. Kidney Int. 2005 Aug;68(2):529-36.
- Abn-CBD
Catalog No.:BCC7011
CAS No.:22972-55-0
- Desmethoxycentaureidin
Catalog No.:BCN5077
CAS No.:22934-99-2
- Neferine
Catalog No.:BCN6338
CAS No.:2292-16-2
- Ginkgolic acid C15:1
Catalog No.:BCN2307
CAS No.:22910-60-7
- R 892
Catalog No.:BCC5992
CAS No.:229030-05-1
- TAK-779
Catalog No.:BCC4137
CAS No.:229005-80-5
- 4-Amino-3,5-dichloropyridine
Catalog No.:BCC8679
CAS No.:22889-78-7
- Silymarin
Catalog No.:BCN6299
CAS No.:22888-70-6
- Famprofazone
Catalog No.:BCC3779
CAS No.:22881-35-2
- 6-Acetonyldihydrochelerythrine
Catalog No.:BCN5076
CAS No.:22864-92-2
- Anisomycin
Catalog No.:BCC7007
CAS No.:22862-76-6
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- Atazanavir sulfate (BMS-232632-05)
Catalog No.:BCC2114
CAS No.:229975-97-7
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
PPAR-gamma agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells.[Pubmed:24132472]
Neurotox Res. 2014 Apr;25(3):311-22.
Tetrabromobisphenol A (2,2-bis(4-hydroxy-3,5-dibromophenyl)propane; TBBPA) is a widely used brominated flame retardant. TBBPA induces neuronal damage, but the mechanism by which this occurs is largely unknown. We studied the possible involvement of peroxisome proliferator-activated receptor gamma (PPAR-gamma) in TBBPA-induced apoptosis and toxicity in mouse primary neuronal cell cultures. TBBPA enhanced both, caspase-3 activity and lactate dehydrogenase (LDH) release in neocortical cells after 6 and 24 h of exposition. These data were supported at the cellular level with Hoechst 33342 staining. Immunoblot analyses showed that, compared with control cells, 10 muM TBBPA decreased the expression of PPAR-gamma protein in neocortical neurons after 1-24 h of exposure. Co-treatment with TBBPA and GW1929 inhibited the TBBPA-induced caspase-3 activity, apoptotic body formation, and LDH release as well as TBBPA-induced decrease in PPAR-gamma protein expression. Thus, our data support neuroprotective potential of PPAR-gamma agonists. The PPAR-gamma antagonist GW9662 prevented the TBBPA-induced decrease in PPAR-gamma protein level, but it potentiated TBBPA-induced apoptotic and neurotoxic effects, which suggest that the mechanism of TBBPA action in neuronal cells is not only PPAR-gamma-dependent. Therefore, further studies of the mechanism of TBBPA action in the nervous system are needed.
The PPAR-gamma antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-gamma in human macrophage polarization.[Pubmed:25972766]
J Inflamm (Lond). 2015 May 3;12:36.
BACKGROUND: The nuclear receptors PPAR-gamma and LXRs regulate macrophage lipid metabolism and macrophage mediated inflammation. We examined the influence of these molecules on macrophage alternative activation, with particular focus on differentiation of "M2c" anti-inflammatory cells. METHODS: We cultured human monocytes in M0, M1, M2a or M2c macrophage differentiating conditions, in the presence or absence of PPAR-gamma and LXR ligands. Flow cytometry was used to analyze membrane expression of phenotypic markers. Basal and LPS-stimulated production of soluble mediators was measured by ELISA. Efferocytosis assays were performed by coincubating monocytes/macrophages with apoptotic neutrophils. RESULTS: We found that PPAR-gamma inhibition, using the PPAR-gamma antagonist GW9662, elicits differentiation of M2c-like (CD206(+) CD163(+) CD16(+)) cells and upregulation of the MerTK/Gas6 axis. Exposure of differentiating macrophages to IFN-gamma, GM-CSF or LPS (M1 conditions), however, hampers GW9662 induction of MerTK and Gas6. When macrophages are differentiated with IL-4 (M2a conditions), addition of GW9662 results into an M2a (CD206(+) CD209(+) CD163(-) MerTK(-)) to M2c (CD206(high) CD209(-) CD163(+) MerTK(+)) polarization shift. Conversely, in the presence of dexamethasone (M2c conditions), the PPAR-gamma agonist rosiglitazone attenuates CD163 and MerTK upregulation. The LXR agonist T0901317 induces MerTK independently of M2c polarization; indeed, CD206, CD163 and CD16 are downregulated. GW9662-differentiated M2c-like cells secrete high levels of Gas6 and low amounts of TNF-alpha and IL-10, mimicking dexamethasone effects in vitro. However, unlike conventional M2c cells, GW9662-differentiated cells do not show enhanced efferocytic ability. CONCLUSIONS: Our results provide new insights into the role of PPAR-gamma and LXR receptors in human macrophage activation and reveal the existence of different patterns regulating MerTK expression. Unexpectedly, PPAR-gamma appears to negatively control the expansion of a discrete subset of M2c-like anti-inflammatory macrophages.
BZ-26, a novel GW9662 derivate, attenuated inflammation by inhibiting the differentiation and activation of inflammatory macrophages.[Pubmed:27710897]
Biomed Pharmacother. 2016 Dec;84:730-739.
Peroxisome proliferator-activated receptor-gamma (PPARgamma) is considered to be an important transcriptional factor in regulation of macrophages differentiation and activation. We have synthesized a series of novel structural molecules based on GW9662's structure (named BZ-24, BZ-25 and BZ-26), and interaction activity was calculated by computational docking. BZ-26 had shown stronger interaction with PPARgamma and had higher transcriptional inhibitory activity of PPARgamma with lower dosage compared with GW9662. BZ-26 was proved to inhibit inflammatory macrophage differentiation. LPS-induced acute inflammation mouse model was applied to demonstrate its anti-inflammatory activity. And the results showed that BZ-26 administration attenuated plasma tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) secretion, which are vital cytokines in acute inflammation. The anti-inflammatory activity was examined in THP-1 cell line, and TNF-alpha, IL-6 and MCP-1, were significantly inhibited. The results of Western blot and luciferase reporter assay indicated that BZ-26 not only inhibited NF-kappaB transcriptional activity, but also abolished LPS-induce nuclear translocation of P65. We also test BZ-26 action in tumor-bearing chronic inflammation mouse model, and BZ-26 was able to alter macrophages phenotype, resulting in antitumor effect. All our data revealed that BZ-26 modulated LPS-induced acute inflammation via inhibiting inflammatory macrophages differentiation and activation, potentially via inhibition of NF-kappaB signal pathway.
Limited Applicability of GW9662 to Elucidate PPARgamma-Mediated Fatty Acid Effects in Primary Human T-Helper Cells.[Pubmed:25054074]
Int J Inflam. 2014;2014:149628.
Synthetic antagonists of the nuclear receptor PPARgamma such as GW9662 are widely used to elucidate receptor-mediated ligand effects. In addition and complementary to recent work, we examined whether GW9662 is suitable to serve for mechanistic investigation in T-helper cells. Human peripheral blood mononuclear cells (PBMC) were preincubated with increasing concentrations of GW9662 (0, 0.4, 2, and 10 mumol/L) 30 min before adding the c9,t11-isomer of conjugated linoleic acid (c9,t11-CLA) as representative of PPARgamma-activating fatty acids with immunomodulatory properties. Corresponding cultures were incubated with GW9662 in the absence of the fatty acid. After 19 h, cells were mitogen stimulated for further 5 h. Subsequently, intracellular IL-2 was measured in CD3(+)CD4(+) lymphocytes by means of flow cytometry. 100 mumol/L c9,t11-CLA reduced the number of T-helper cells expressing IL-2 by 68%. GW9662 failed to abrogate this fatty acid effect, likely due to the fact that the compound exerted an own inhibitory effect on IL-2 production. Moreover, GW9662 dose-dependently induced cell death in human leukocytes. These results suggest that application of GW9662 is not conducive in this experimental setting.
GW9662, a potent antagonist of PPARgamma, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation.[Pubmed:15533890]
Br J Pharmacol. 2004 Dec;143(8):933-7.
Peroxisome proliferator-activated receptor gamma (PPARgamma), a member of the nuclear receptor superfamily, is activated by several compounds, including the thiazolidinediones. In addition to being a therapeutic target for obesity, hypolipidaemia and diabetes, perturbation of PPARgamma signalling is now believed to be a strategy for treatment of several cancers, including breast. Although differential expression of PPARgamma is observed in tumours compared to normal tissues and PPARgamma agonists have been shown to inhibit tumour cell growth and survival, the interdependence of these observations is unclear. This study demonstrated that the potent, irreversible and selective PPARgamma antagonist GW9662 prevented activation of PPARgamma and inhibited growth of human mammary tumour cell lines. Controversially, GW9662 prevented rosiglitazone-mediated PPARgamma activation, but enhanced rather than reversed rosiglitazone-induced growth inhibition. As such, these data support the existence of PPARgamma-independent pathways and question the central belief that PPARgamma ligands mediate their anticancer effects via activation of PPARgamma.
Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662.[Pubmed:12022867]
Biochemistry. 2002 May 28;41(21):6640-50.
In the course of a high throughput screen to search for ligands of peroxisome proliferator activated receptor-gamma (PPARgamma), we identified GW9662 using a competition binding assay against the human ligand binding domain. GW9662 had nanomolar IC(50) versus PPARgamma and was 10- and 600-fold less potent in binding experiments using PPARalpha and PPARdelta, respectively. Pretreatment of all three PPARs with GW9662 resulted in the irreversible loss of ligand binding as assessed by scintillation proximity assay. Incubation of PPAR with GW9662 resulted in a change in the absorbance spectra of the receptors consistent with covalent modification. Mass spectrometric analysis of the PPARgamma ligand binding domain treated with GW9662 established Cys(285) as the site of covalent modification. This cysteine is conserved among all three PPARs. In cell-based reporter assays, GW9662 was a potent and selective antagonist of full-length PPARgamma. The functional activity of GW9662 as an antagonist of PPARgamma was confirmed in an assay of adipocyte differentiation. GW9662 showed essentially no effect on transcription when tested using both full-length PPARdelta and PPARalpha. Time-resolved fluorescence assays of ligand-modulated receptor heterodimerization, coactivator binding, and corepressor binding were consistent with the effects observed in the reporter gene assays. Control activators increased PPAR:RXR heterodimer formation and coactivator binding to both PPARgamma and PPARdelta. Corepressor binding was decreased. In the case of PPARalpha, GW9662 treatment did not significantly increase heterodimerization and coactivator binding or decrease corepressor binding. The experimental data indicate that GW9662 modification of each of the three PPARs results in different functional consequences. The selective and irreversible nature of GW9662 treatment, and the observation that activity is maintained in cell culture experiments, suggests that this compound may be a useful tool for elucidation of the role of PPARgamma in biological processes.
IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1.[Pubmed:11226258]
Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2443-8.
IL-4 is a pleiotropic immune cytokine secreted by activated T(H)2 cells that inhibits bone resorption both in vitro and in vivo. The cellular targets of IL-4 action as well as its intracellular mechanism of action remain to be determined. We show here that IL-4 inhibits receptor activator of NF-kappaB ligand-induced osteoclast differentiation through an action on osteoclast precursors that is independent of stromal cells. Interestingly, this inhibitory effect can be mimicked by both natural as well as synthetic peroxisome proliferator-activated receptor gamma1 (PPARgamma1) ligands and can be blocked by the irreversible PPARgamma antagonist GW 9662. These findings suggest that the actions of IL-4 on osteoclast differentiation are mediated by PPARgamma1, an interpretation strengthened by the observation that IL-4 can activate a PPARgamma1-sensitive luciferase reporter gene in RAW264.7 cells. We also show that inhibitors of enzymes such as 12/15-lipoxygenase and the cyclooxygenases that produce known PPARgamma1 ligands do not abrogate the IL-4 effect. These findings, together with the observation that bone marrow cells from 12/15-lipoxygenase-deficient mice retain sensitivity to IL-4, suggest that the cytokine may induce novel PPARgamma1 ligands. Our results reveal that PPARgamma1 plays an important role in the suppression of osteoclast formation by IL-4 and may explain the beneficial effects of the thiazolidinedione class of PPARgamma1 ligands on bone loss in diabetic patients.


