GBR 12783 dihydrochloridePotent, selective dopamine uptake inhibitor CAS# 67469-75-4 |
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 67469-75-4 | SDF | Download SDF |
| PubChem ID | 24744862 | Appearance | Powder |
| Formula | C28H34Cl2N2O | M.Wt | 485.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water with gentle warming | ||
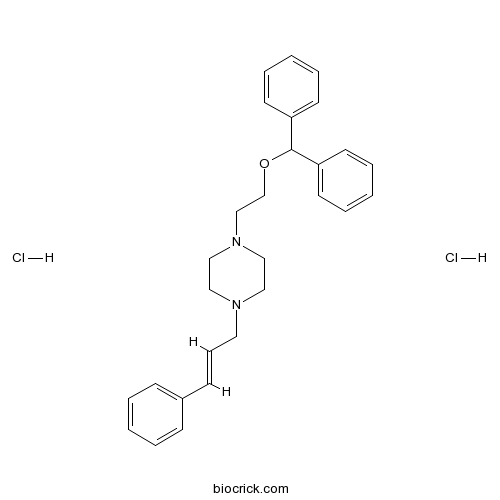
| Chemical Name | 1-(2-benzhydryloxyethyl)-4-[(E)-3-phenylprop-2-enyl]piperazine;dihydrochloride | ||
| SMILES | C1CN(CCN1CCOC(C2=CC=CC=C2)C3=CC=CC=C3)CC=CC4=CC=CC=C4.Cl.Cl | ||
| Standard InChIKey | HJDXBLLTRMCYNC-JFXLULTRSA-N | ||
| Standard InChI | InChI=1S/C28H32N2O.2ClH/c1-4-11-25(12-5-1)13-10-18-29-19-21-30(22-20-29)23-24-31-28(26-14-6-2-7-15-26)27-16-8-3-9-17-27;;/h1-17,28H,18-24H2;2*1H/b13-10+;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A very potent and selective inhibitor of dopamine uptake (IC50 for inhibition of [3H]-dopamine uptake in rat striatal synaptosomes is 1.8 nM). |

GBR 12783 dihydrochloride Dilution Calculator

GBR 12783 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0597 mL | 10.2987 mL | 20.5973 mL | 41.1946 mL | 51.4933 mL |
| 5 mM | 0.4119 mL | 2.0597 mL | 4.1195 mL | 8.2389 mL | 10.2987 mL |
| 10 mM | 0.206 mL | 1.0299 mL | 2.0597 mL | 4.1195 mL | 5.1493 mL |
| 50 mM | 0.0412 mL | 0.206 mL | 0.4119 mL | 0.8239 mL | 1.0299 mL |
| 100 mM | 0.0206 mL | 0.103 mL | 0.206 mL | 0.4119 mL | 0.5149 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vanoxerine
Catalog No.:BCC5130
CAS No.:67469-69-6
- GBR 13069 dihydrochloride
Catalog No.:BCC6640
CAS No.:67469-45-8
- Amorolfine
Catalog No.:BCC8819
CAS No.:67467-83-8
- Isoasatone A
Catalog No.:BCN7762
CAS No.:67451-73-4
- Fmoc-Cys(tBu)-OH
Catalog No.:BCC3478
CAS No.:67436-13-9
- Isorhamnetin-3-O-galactoside
Catalog No.:BCC8190
CAS No.:6743-92-6
- (±)-Blebbistatin
Catalog No.:BCC7169
CAS No.:674289-55-5
- Z-D-His-OH
Catalog No.:BCC2767
CAS No.:67424-93-5
- 3-O-Acetyl-11-keto-beta-boswellic acid
Catalog No.:BCN1381
CAS No.:67416-61-9
- H-D-Lys-OMe.2HCl
Catalog No.:BCC2680
CAS No.:67396-08-1
- Drospirenone
Catalog No.:BCC4493
CAS No.:67392-87-4
- Catalposide
Catalog No.:BCN4225
CAS No.:6736-85-2
- Vanoxerine dihydrochloride
Catalog No.:BCC5129
CAS No.:67469-78-7
- GBR 12935 dihydrochloride
Catalog No.:BCC5380
CAS No.:67469-81-2
- DIDS
Catalog No.:BCC7942
CAS No.:67483-13-0
- Fuegin
Catalog No.:BCN5809
CAS No.:6750-10-3
- Eupatolide
Catalog No.:BCN7345
CAS No.:6750-25-0
- Arnidiol
Catalog No.:BCN3810
CAS No.:6750-30-7
- Spathulenol
Catalog No.:BCN4227
CAS No.:6750-60-3
- 4-Methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile
Catalog No.:BCC8710
CAS No.:675126-26-8
- Thapsigargin
Catalog No.:BCC6952
CAS No.:67526-95-8
- Alpha-caryophyllene
Catalog No.:BCN3877
CAS No.:6753-98-6
- Helenalin
Catalog No.:BCN8073
CAS No.:6754-13-8
- Dehydrotumulosic acid
Catalog No.:BCN3740
CAS No.:6754-16-1
Evidence for the sequential formation of two complexes between an uptake inhibitor, GBR 12783 [1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-2-propenyl)piperazine], and the neuronal transporter of dopamine.[Pubmed:9886093]
J Neurochem. 1999 Jan;72(1):396-404.
Incubation of a crude synaptosomal fraction from rat striatum with GBR 12783 at 37 degrees C produced an inhibition of the specific uptake of [3H]dopamine that increased with time. The inhibition increased when GBR 12783 was present during preincubation and incubation (IC50 = 1.85+/-0.1 nM) instead of incubation alone (IC50 = 25+/-3.5 nM). Time-course studies of uptake inhibition demonstrated that a first collision transporter-inhibitor complex (TI) was formed immediately after addition of GBR 12783 so that the initial uptake velocity (V0) decreased for increasing concentrations of inhibitor (Ki > or = 20 nM). TI slowly isomerized to a more stable complex TI* (Ki* < or = 5 nM) with a value of t1/2 = 20-270 s. Fits of data to model 2 in which the steady-state uptake (VS) is set to zero were generally preferred, suggesting that formation of TI* could tend to irreversibility, as a consequence of a very low reverse isomerization. As expected, k, V0, and VS tended to steady-state values in an asymptotic manner for high concentrations of GBR 12783. GBR 12783 at 2.5 nM produced a mixed inhibition of the uptake, with an increase in KM and a decrease in Vmax; these effects were improved for 10 nM GBR 12783 and at 20 degrees C. These results are discussed in relation to previous data concerning [3H]GBR 12783 binding. The present work gives the first experimental demonstration that dopamine uptake blockers can act according to a two-step mechanism of inhibition; this is of great interest, because these inhibitors can oppose the effects of cocaine or amphetamine on the transporter according to a reaction that is partly nondependent on the concentration of the abused agent.
Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter.[Pubmed:1406597]
Mol Pharmacol. 1992 Sep;42(3):383-90.
We have screened a human substantia nigra cDNA library with probes derived from the rat dopamine transporter. A 3.5-kilobase cDNA clone was isolated and its corresponding gene was located on the distal end of chromosome 5 (5p15.3). This human clone codes for a 620-amino acid protein with a calculated molecular weight of 68,517. Hydropathicity analysis suggests the presence of 12 putative transmembrane domains, a characteristic feature of sodium-dependent neurotransmitter carriers. The rat and the human dopamine transporters are 92% homologous. When permanently expressed in mouse fibroblast Ltk- cells, the human clone is able to induce a saturable, time- and sodium-dependent, dopamine uptake. This transport is blocked by psychostimulant drugs (cocaine, l- and d-amphetamine, and phenyclidine), neurotoxins (6-hydroxydopamine and N-methyl-4-phenylpyridine (MPP))+), neurotransmitters (epinephrine, norepinephrine, gamma-aminobutyric acid, and serotonin), antidepressants (amitriptyline, bupropion, desipramine, mazindol, nomifensine, and nortriptyline), and various uptake inhibitors (mazindol, GBR 12783, GBR 12909, and amfonelic acid). The rank orders of the Ki values of these substances at the human and the rat dopamine transporters are highly correlated (r = 0.998). The cloning of DNA human dopamine transporter gene has allowed establishment of a cell line stably expressing the human dopamine transporter and, for the first time, an extensive characterization of its pharmacology. Furthermore, these newly developed tools will help in the study of the regulation of dopamine transport in humans and in the clarification of the potential role of the dopamine transporter in a variety of disease states.
In vivo labelling of the neuronal dopamine uptake complex in the mouse striatum by [3H]GBR 12783.[Pubmed:1350989]
Eur J Pharmacol. 1992 Jan 7;210(1):77-84.
Various characteristics of the in vivo striatal binding of [3H]GBR 12783 (1-[2-(diphenylmethoxy)-ethyl]-4-(3-phenyl-1[3H]-2-propenyl)pipera zine), a specific ligand of the neuronal dopamine uptake complex, were determined in mice. Increasing doses of the ligand revealed the saturability of the binding at a single site with half-maximal saturation at a dose of approximately 7 mumol/kg and an apparent maximal number of binding sites (Bmax) of 12.8 pmol/mg protein in striatum. Specific binding was prevented by various dopamine uptake blockers, pyrovalerone, GBR 13069, GBR 12783, N-[1-2-benzo(b)thiophenyl)cyclohexyl] piperidine, cocaine, methylphenidate and was inhibited in a stereoselective manner by the enantiomers of nomifensine. Other drugs which are not dopamine uptake blockers either did not modify [3H]GBR 12783 binding (the diphenylbutylpiperazine derivative flupenthixol) or increased it (the diphenylpiperazine derivative flunarizine or the chemically unrelated compounds fenfluramine and SKF 525A). A close correlation was found between occupancy of the striatal [3H]GBR 12783 binding site and the stimulant locomotor effect of the drug. A similar specific striatal binding of [3H]GBR 12783 was evidenced in both NMRI and CD1 strains. It was concluded that [3H]GBR 12783 administered in vivo provides a measure of the density of dopamine uptake sites in mouse striatum.
GBR 12783, a potent and selective inhibitor of dopamine uptake: biochemical studies in vivo and ex vivo.[Pubmed:3754516]
Eur J Pharmacol. 1986 Feb 18;121(2):199-209.
The effects of GBR 12783, an aryl 1,4-dialk(en)ylpiperazine derivative, were studied on the in vivo and ex vivo neuronal uptake of dopamine (DA), norepinephrine (NE) and serotonin (5HT). The drug inhibited potently (IC50 = 1.8 nM) and competitively the [3H]DA uptake by rat striatal synaptosomes. It produced significant [14C]DA release only at much higher concentrations (in the micromolar range). Depending on the animal species (rat or mouse) and the experimental conditions, GBR 12783 was 18-90 times and 85-300 times less effective against NE and 5HT uptake respectively than against DA uptake. In synaptosomes preloaded with [3H]DA, GBR 12783 added to the superfusion medium prevented the (+)amphetamine-induced DA release. The total binding of [3H]GBR 12783 to a membranal fraction prepared from striatum was lower than the binding to a synaptosomal fraction, suggesting its entry in synaptosomes. In addition, the concentration-dependent release of [3H]DA produced by GBR 12783 from a striatal vesicular fraction may account for the synaptosomal DA release promoted by micromolar concentrations of the drug. In ex vivo experiments, the ID50 for DA uptake inhibition (30 min after i.p. administration) was 8.1 mg/kg. After a dose of 10 mg/kg i.p., the striatal DA uptake inhibition occurred quickly (less than 10 min) and was long-lasting (greater than 5 h). The specificity of the drug for the DA uptake relative to NE and 5HT uptakes was also seen after i.p. administration of GBR 12783.


