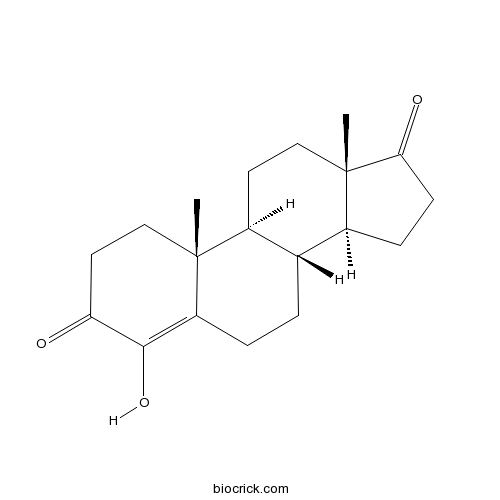
FormestaneType I steroidal aromatase inhibitor CAS# 566-48-3 |
- LY2801653 dihydrochloride
Catalog No.:BCC1721
CAS No.:1206801-37-7
- c-Met inhibitor 1
Catalog No.:BCC1488
CAS No.:1357072-61-7
- Sunitinib malate
Catalog No.:BCC3664
CAS No.:341031-54-7
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 566-48-3 | SDF | Download SDF |
| PubChem ID | 11273 | Appearance | Powder |
| Formula | C19H26O3 | M.Wt | 302.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | (8R,9S,10R,13S,14S)-4-hydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,17-dione | ||
| SMILES | CC12CCC(=O)C(=C1CCC3C2CCC4(C3CCC4=O)C)O | ||
| Standard InChIKey | OSVMTWJCGUFAOD-KZQROQTASA-N | ||
| Standard InChI | InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-13,22H,3-10H2,1-2H3/t11-,12-,13-,18+,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Formestane Dilution Calculator

Formestane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3068 mL | 16.5338 mL | 33.0677 mL | 66.1354 mL | 82.6692 mL |
| 5 mM | 0.6614 mL | 3.3068 mL | 6.6135 mL | 13.2271 mL | 16.5338 mL |
| 10 mM | 0.3307 mL | 1.6534 mL | 3.3068 mL | 6.6135 mL | 8.2669 mL |
| 50 mM | 0.0661 mL | 0.3307 mL | 0.6614 mL | 1.3227 mL | 1.6534 mL |
| 100 mM | 0.0331 mL | 0.1653 mL | 0.3307 mL | 0.6614 mL | 0.8267 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective, irreversible and competitive type I steroidal aromatase inhibitor. Shows minor androgenic activity. Reduces plasma levels of estrogen and shows antitumor effects in vivo. Orally active.
- 7Beta-Hydroxycholesterol
Catalog No.:BCN2751
CAS No.:566-27-8
- 7-Keto-dehydroepiandrosterone
Catalog No.:BCC8780
CAS No.:566-19-8
- 3-Hydroxy-2-phenyl-propanamide
Catalog No.:BCN3905
CAS No.:56598-62-0
- PIT
Catalog No.:BCC7151
CAS No.:56583-49-4
- (-)-Bornyl acetate
Catalog No.:BCN2636
CAS No.:5655-61-8
- Cyclo(Pro-Val)
Catalog No.:BCN2420
CAS No.:5654-87-5
- Cyclo(Pro-Leu)
Catalog No.:BCN2426
CAS No.:5654-86-4
- Cyclo(Tyr-Pro)
Catalog No.:BCN2421
CAS No.:5654-84-2
- 2-Hydroxy-3,4-dimethoxybenzoic acid
Catalog No.:BCN6535
CAS No.:5653-46-3
- Angelic acid
Catalog No.:BCN3410
CAS No.:565-63-9
- Steppogenin
Catalog No.:BCN5760
CAS No.:56486-94-3
- 4,9-Dihydroxy-alpha-lapachone
Catalog No.:BCN5758
CAS No.:56473-67-7
- BOP reagent
Catalog No.:BCC2807
CAS No.:56602-33-6
- H-D-Phe(4-NO2)-OH
Catalog No.:BCC3274
CAS No.:56613-61-7
- D-Phenylglycinol
Catalog No.:BCC2712
CAS No.:56613-80-0
- Z-Arg-OH.HCl
Catalog No.:BCC3061
CAS No.:56672-63-0
- Batatasin III
Catalog No.:BCN3596
CAS No.:56684-87-8
- 2-Benzylacrylic acid
Catalog No.:BCC8564
CAS No.:5669-19-2
- Orteronel
Catalog No.:BCC1823
CAS No.:566939-85-3
- (+/-)-Vestitol
Catalog No.:BCN6814
CAS No.:56701-24-7
- Z-Glu-OMe
Catalog No.:BCC2779
CAS No.:5672-83-3
- Icariside I
Catalog No.:BCN3463
CAS No.:56725-99-6
- 1-Methoxyallocryptopine
Catalog No.:BCN7454
CAS No.:56743-52-3
- Phenylalanine betaine
Catalog No.:BCN5761
CAS No.:56755-22-7
Profiling of urinary formestane and confirmation by isotope ratio mass spectrometry.[Pubmed:23933120]
Steroids. 2013 Nov;78(11):1103-9.
Formestane (F, androst-4-en-4-ol-3,17-dione) is an irreversible aromatase inhibitor with the ability to suppress the estrogen production from anabolic steroids. Consequently, F is mentioned on the World Anti-Doping Agency (WADA) prohibited list and because studies have shown that F is produced endogenously in small amounts, a threshold for urinary excreted F of 150 ng/mL was introduced. Lower concentrations could be due to endogenous production and need further investigation to prove the exact origin through determination of the carbon isotope ratio. However, because the current screening methods are a lot more sensitive, F is detected in practically every urine sample. A strict implementation of this WADA rule would imply that almost every urine sample needs additional investigation to verify an exogenous or endogenous origin. The main aim of this study was to propose and introduce a lower concentration limit of 25 ng/mL beneath which the detected F is considered as being endogenous and no further investigation is needed. The data presented in this paper suggests that this threshold provides a good balance between a sufficiently large detection window and not having to perform isotope ratio mass spectrometry (IRMS) analyses on negative urine samples.
Synthesis and bioconversions of formestane.[Pubmed:24074257]
J Nat Prod. 2013 Oct 25;76(10):1966-9.
In an effort to generate new steroidal aromatase inhibitors, Formestane (4-hydroxyandrost-4-ene-3,17-dione) (1) was biotransformed by Rhizopus oryzae to yield the known 4beta,5alpha-dihydroxyandrostane-3,17-dione as the major product (5) and bioconverted by Beauveria bassiana to afford the known reduced 4,17beta-dihydroxyandrost-4-en-3-one (6) and 3alpha,17beta-dihydroxy-5beta-androstan-4-one (7) and the new 4,11alpha,17beta-trihydroxyandrost-4-en-3-one (8). All the metabolites showed more potent activities than their parent congener in the aromatase and MCF-7 breast cancer assays. The bioactivities and structural elucidation of these metabolites as well as the semisynthesis of Formestane (1) from testosterone (2) are reported herein.
Interactions of an anticancer drug Formestane with single and double stranded DNA at physiological conditions.[Pubmed:26036658]
J Photochem Photobiol B. 2015 Aug;149:27-36.
Mode of interactions of anticancer drug Formestane (FMT) with single and double stranded DNA has been investigated at different temperatures and at two physiological pH values i.e. 7.4 (human blood pH) and 4.7 (stomach pH). Fluorescence spectroscopy, UV-Vis spectroscopy, cyclic voltammetry and square wave voltammetry were employed to probe the interaction between FMT and DNA. The observed fluorescence quenching of dsDNA-ethidium bromide system by the anticancer drug FMT confirmed the intercalative mode of binding between the FMT and dsDNA. The absorption spectra and voltammetric results indicate FMT gets intercalated between dsDNA bases and the strength of interaction is independent on the ionic strength. Comparison of the mode of interaction of FMT with dsDNA and ssDNA was discussed. The calculated binding constants for FMT-dsDNA and FMT-ssDNA complexes at pH 7.4 were found to be 1.52x10(5)M(-1) and 1.24x10(6)M(-1), respectively. Stoichiometric coefficients and thermodynamic parameters of FMT-dsDNA and FMT-ssDNA complexes were evaluated. The association between the anticancer drug FMT with DNA is maximum at pH 7.4 which depicts the most stable complexes are formed at human blood pH. The decrease in peak current of FMT resulting from its interaction with DNA was employed for determination of dsDNA and ssDNA concentration at physiological conditions.
Detection of formestane abuse by mass spectrometric techniques.[Pubmed:25516450]
Drug Test Anal. 2014 Nov-Dec;6(11-12):1133-40.
Formestane (4-hydroxy-androstenedione) is an aromatase inhibitor prohibited in sports and included, since 2004, in the list of prohibited substances updated yearly by the World Anti-Doping Agency (WADA). Since the endogenous production of Formestane has been described, it is mandatory for the anti-doping laboratories to use isotope ratio mass spectrometry (IRMS) to establish the exogenous origin before issuing an adverse analytical finding. The described IRMS methods for Formestane detection are time-consuming, requiring usually two consecutive liquid chromatographic sample purifications in order to have final extracts of adequate purity before the mass spectrometric analysis. After establishing a procedure for the determination of the origin of Formestane by IRMS without the need of derivatization, and integrated in the overall analytical strategy of the laboratory for pseudo-endogenous steroids, a mass spectrometric analysis by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS) of Formestane metabolites was carried out in order to investigate whether other biomarkers of Formestane abuse could be integrated in order to avoid time-consuming and expensive IRMS confirmations for Formestane. From the metabolic studies performed, the inclusion of 3beta,4alpha-dihydroxy-5alpha-androstan-17-one (4alpha-hydroxy-epiandosterone) in the routine GC-MS procedures has demonstrated to be diagnostic in order to reduce the number of unnecessary confirmations of the endogenous origin of Formestane.


