Divalproex SodiumEpilepsy/migraines/bipolar disorder medicine CAS# 76584-70-8 |
- Nepicastat (SYN-117) HCl
Catalog No.:BCC2286
CAS No.:170151-24-3
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Mildronate
Catalog No.:BCC2289
CAS No.:76144-81-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 76584-70-8 | SDF | Download SDF |
| PubChem ID | 23663956 | Appearance | Powder |
| Formula | C16H31NaO4 | M.Wt | 310.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | sodium;2-propylpentanoate;2-propylpentanoic acid | ||
| SMILES | [Na+].CCCC(CCC)C(O)=O.CCCC(CCC)C([O-])=O | ||
| Standard InChIKey | MSRILKIQRXUYCT-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/2C8H16O2.Na/c2*1-3-5-7(6-4-2)8(9)10;/h2*7H,3-6H2,1-2H3,(H,9,10);/q;;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Divalproex Sodium Dilution Calculator

Divalproex Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2215 mL | 16.1077 mL | 32.2155 mL | 64.4309 mL | 80.5386 mL |
| 5 mM | 0.6443 mL | 3.2215 mL | 6.4431 mL | 12.8862 mL | 16.1077 mL |
| 10 mM | 0.3222 mL | 1.6108 mL | 3.2215 mL | 6.4431 mL | 8.0539 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6443 mL | 1.2886 mL | 1.6108 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6443 mL | 0.8054 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
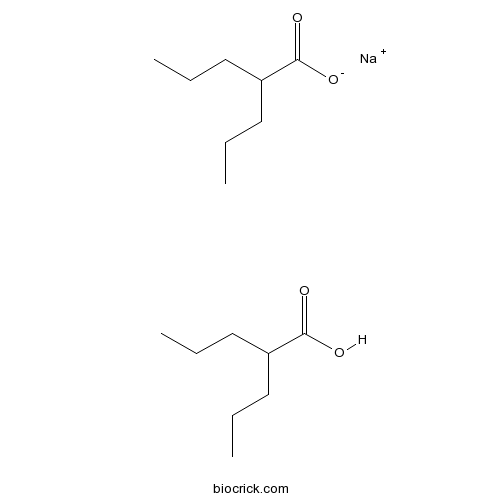
Divalproex sodium (USAN) consists of a compound of sodium valproate and valproic acid in a 1:1 molar relationship in an enteric coated form.[1] Its chief use in medicine is as a treatment for bipolar disorder, epilepsy and in the prevention of migraines.
- SR 202
Catalog No.:BCC7243
CAS No.:76541-72-5
- Heraclenol 3'-O-[beta-D-apiofuranosyl-(1-6)-beta-D-glucopyranoside]
Catalog No.:BCN1362
CAS No.:765316-44-7
- 1-2-Cyclohexanedione
Catalog No.:BCN2265
CAS No.:765-87-7
- 10-Hydroxy-2-decenoic acid
Catalog No.:BCN2654
CAS No.:765-01-5
- 15,16-Dinor-8(17),11-labdadien-13-one
Catalog No.:BCN4312
CAS No.:76497-69-3
- Ligustrazine Hydrochloride
Catalog No.:BCN1009
CAS No.:76494-51-4
- 8-Acetoxy-15,16-epoxy-8,9-secolabda-13(16),14-diene-7,9-dione
Catalog No.:BCN7409
CAS No.:76475-32-6
- Croverin
Catalog No.:BCN2518
CAS No.:76475-17-7
- Galeopsin
Catalog No.:BCN7358
CAS No.:76475-16-6
- Dihydrocurcumin
Catalog No.:BCN6297
CAS No.:76474-56-1
- Morachalcone A
Catalog No.:BCN4311
CAS No.:76472-88-3
- Kuwanon H
Catalog No.:BCN2945
CAS No.:76472-87-2
- OR-486
Catalog No.:BCC5661
CAS No.:7659-29-2
- 3-Ethyl-4-methyl-3-pyrrolin-2-one
Catalog No.:BCC8632
CAS No.:766-36-9
- Imidazo[1,2-b]pyridazine
Catalog No.:BCC9001
CAS No.:766-55-2
- beta-D-Fructopyranose
Catalog No.:BCC8176
CAS No.:7660-25-5
- BAM 22P
Catalog No.:BCC5797
CAS No.:76622-26-9
- Detomidine
Catalog No.:BCC4079
CAS No.:76631-46-4
- RU 26752
Catalog No.:BCC7531
CAS No.:76676-33-0
- RU 28318, potassium salt
Catalog No.:BCC7146
CAS No.:76676-34-1
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- 1-Deacetylnimbolinin B
Catalog No.:BCN4313
CAS No.:76689-98-0
- Mallorepine
Catalog No.:BCN4317
CAS No.:767-98-6
- Decumbenine
Catalog No.:BCC8312
CAS No.:76733-83-0
Development of fixed dose combination tablets of aripiprazole plus divalproex sodium and their simultaneous determination using HPLC-UV.[Pubmed:26727505]
Drug Dev Ind Pharm. 2016 Sep;42(9):1393-405.
A vast majority of psychiatric patients are effectively treated with combination of drugs to improve efficacy and adherence, but due to limited research and development in fixed dose combination (FDC) in psychiatry, these products are not commonly available. The aim of this study is to prepare cost effective FDC tablets containing aripiprazole and Divalproex Sodium. Two batches of fixed dose combination tablets, FDC1 and FDC2, were successfully prepared using wet granulation technique. Furthermore, aripiprazole tablets A1 and A2 and divalproex tablets D1 were also formulated as reference to compare the in vitro availability profile. An accurate and simple isocratic HPLC method was established and validated for the simultaneous quantification of aripiprazole and valproic acid in the FDC tablets. A reversed-phase C18 (250 x 4.6 mm) column in isocratic mode was used. The mobile phase consisted of acetonitrile and 0.32% KH2PO4 (60:40, v/v), flow rate was set at 1.0 mL/min and the detection was performed at 210 nm. Average percent recoveries of aripiprazole and valproic acid were 96.0 and 95.5%, respectively, meeting the official requirements. The newly developed FDC product may be used for the better therapeutic outcomes of combined use of aripiprazole and valproic acid, which may improve patient adherence.
Safety and effectiveness of divalproex sodium extended release containing regimen in Indian patients with bipolar I disorder in continuation phase: Results of EASED registry.[Pubmed:27025469]
Asian J Psychiatr. 2016 Apr;20:32-8.
The study was conducted to evaluate the safety and effectiveness of Divalproex Sodium XR containing regimen in patients with bipolar disorder (BPD) who are in continuation phase. It was an open-label, prospective, observational study conducted from July 2010 to December 2011 at 48 sites across India. Adult patients with bipolar I disorder of manic or mixed type fulfilling the DSM-IV criteria and who were in the continuation phase were included. Safety (primary outcome) was assessed by incidence of treatment emergent adverse events (AEs). Effectiveness (secondary outcome), was evaluated by proportion of patients who did not have a relapse, change in Clinical Global Impression Score-BP version-Severity of Illness (CGI-BP) and Young's Mania Rating Scale (YMRS) score. Data was recorded at three visits: visit-1 (baseline), visit-2 (end of 2 months +/- 7 days) and visit-3 (end of 4 months +/- 14 days), and summarised using descriptive statistics. p<0.05 was considered statistically significant. A total of 489 and 468 patients were included in the safety and effectiveness analyses, respectively. Of the 66 AEs reported, 57 (89.0%) were mild and 7 (10.9%) were moderate (data missing for 2 events). In total, 75.0% (48/64) of the AEs were related to the study drug. No serious AEs reported (N=64). No relapse observed in 93.3% of patients. There was a significant (p<0.0001) reduction in the YMRS and CGI-BP scores from baseline to visit-3. Our study confirms the results of earlier studies in terms of good tolerability and effectiveness of Divalproex Sodium XR containing regimen in this study population.
Release property study on the novel divalproex sodium enteric-coated capsules.[Pubmed:27275109]
Saudi Pharm J. 2016 May;24(3):245-9.
In the present study, a novel Divalproex Sodium (DS) enteric-coated capsule was prepared, and high performance liquid chromatography (HPLC) assay method for DS was developed. Their uniformity, release curve and release characteristics in different solvents were examined. The release studies were performed using marketed sample as a reference and data were analyzed in terms of cumulative release amounts as a function of time. It was demonstrated by the results that assay developed was specific, rapid and reliable, which can be used to determine DS in vitro accurately, and our developed samples were similar to reference preparation in in vitro release characteristics. The release characteristics of different batches of samples were quite similar to each other, and the total release percents of DS from enteric-coated capsule were within 0-10% in HCl, and reached close to 100% in phosphate buffer. Similarity factors (f 2) of three batches between two preparations were all higher than 50. The developed enteric-coated capsule may be a promising alternative dosage form for treatment of related diseases.


