Dibucaine (Cinchocaine) HClCAS# 61-12-1 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
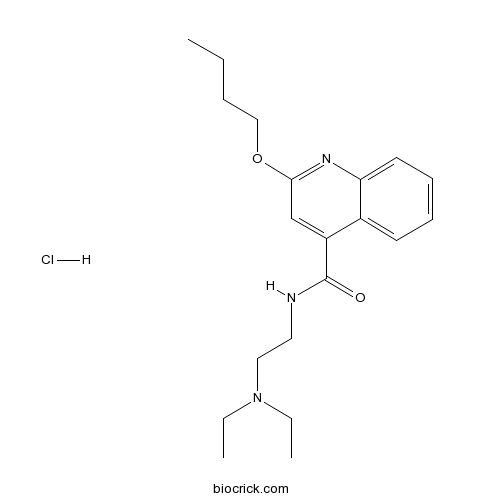
| Cas No. | 61-12-1 | SDF | Download SDF |
| PubChem ID | 521951 | Appearance | Powder |
| Formula | C20H30ClN3O2 | M.Wt | 379.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | 2-butoxy-N-[2-(diethylamino)ethyl]quinoline-4-carboxamide;hydrochloride | ||
| SMILES | CCCCOC1=NC2=CC=CC=C2C(=C1)C(=O)NCCN(CC)CC.Cl | ||
| Standard InChIKey | IVHBBMHQKZBJEU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H29N3O2.ClH/c1-4-7-14-25-19-15-17(16-10-8-9-11-18(16)22-19)20(24)21-12-13-23(5-2)6-3;/h8-11,15H,4-7,12-14H2,1-3H3,(H,21,24);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dibucaine (Cinchocaine) HCl Dilution Calculator

Dibucaine (Cinchocaine) HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6321 mL | 13.1607 mL | 26.3213 mL | 52.6427 mL | 65.8033 mL |
| 5 mM | 0.5264 mL | 2.6321 mL | 5.2643 mL | 10.5285 mL | 13.1607 mL |
| 10 mM | 0.2632 mL | 1.3161 mL | 2.6321 mL | 5.2643 mL | 6.5803 mL |
| 50 mM | 0.0526 mL | 0.2632 mL | 0.5264 mL | 1.0529 mL | 1.3161 mL |
| 100 mM | 0.0263 mL | 0.1316 mL | 0.2632 mL | 0.5264 mL | 0.658 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dibucaine(Cinchocaine) HCl is an amide local anesthetic. I
- 2-Methoxycinnamic acid
Catalog No.:BCN5038
CAS No.:6099-03-2
- Geraniin
Catalog No.:BCN2402
CAS No.:60976-49-0
- Bz-Glu-OH
Catalog No.:BCC2922
CAS No.:6094-36-6
- YZ9
Catalog No.:BCC8001
CAS No.:6093-71-6
- 6-Hydroxycoumarin
Catalog No.:BCC9207
CAS No.:6093-68-1
- H-DL-Pro-OH
Catalog No.:BCC3026
CAS No.:609-36-9
- PIR 3.5
Catalog No.:BCC6128
CAS No.:6088-51-3
- Ligularidine
Catalog No.:BCN2141
CAS No.:60872-63-1
- Crotaverrine
Catalog No.:BCN2142
CAS No.:60827-69-2
- Esculentoside B
Catalog No.:BCN5011
CAS No.:60820-94-2
- Apremilast (CC-10004)
Catalog No.:BCC2273
CAS No.:608141-41-9
- 4-Methoxy-1-methoxycarbonyl-beta-carboline
Catalog No.:BCN1401
CAS No.:60807-25-2
- Adenosine 5'-monophosphate
Catalog No.:BCC8809
CAS No.:61-19-8
- Papaverine Hydrochloride
Catalog No.:BCC8348
CAS No.:61-25-6
- Mefenamic Acid
Catalog No.:BCC4433
CAS No.:61-68-7
- Phenylephrine HCl
Catalog No.:BCC4335
CAS No.:61-76-7
- 4-Aminohippuric Acid
Catalog No.:BCC4753
CAS No.:61-78-9
- Zoxazolamine
Catalog No.:BCC4751
CAS No.:61-80-3
- H-Leu-OH
Catalog No.:BCC2968
CAS No.:61-90-5
- Tenuazonic acid
Catalog No.:BCN1859
CAS No.:610-88-8
- Isoacetovanillone
Catalog No.:BCN7166
CAS No.:6100-74-9
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
- Ferulamide
Catalog No.:BCN4129
CAS No.:61012-31-5
- c-JUN peptide
Catalog No.:BCC8085
CAS No.:610273-01-3
Natural abundance (14)N and (15)N solid-state NMR of pharmaceuticals and their polymorphs.[Pubmed:27314503]
Phys Chem Chem Phys. 2016 Jun 29;18(26):17713-30.
(14)N ultra-wideline (UW), (1)H{(15)N} indirectly-detected HETCOR (idHETCOR) and (15)N dynamic nuclear polarization (DNP) solid-state NMR (SSNMR) experiments, in combination with plane-wave density functional theory (DFT) calculations of (14)N EFG tensors, were utilized to characterize a series of nitrogen-containing active pharmaceutical ingredients (APIs), including HCl salts of scopolamine, alprenolol, isoprenaline, acebutolol, dibucaine, nicardipine, and ranitidine. A case study applying these methods for the differentiation of polymorphs of bupivacaine HCl is also presented. All experiments were conducted upon samples with naturally-abundant nitrogen isotopes. For most of the APIs, it was possible to acquire frequency-stepped UW (14)N SSNMR spectra of stationary samples, which display powder patterns corresponding to pseudo-tetrahedral (i.e., RR'R''NH(+) and RR'NH2(+)) or other (i.e., RNH2 and RNO2) nitrogen environments. Directly-excited (14)N NMR spectra were acquired using the WURST-CPMG pulse sequence, which incorporates WURST (wideband, uniform rate, and smooth truncation) pulses and a CPMG (Carr-Purcell Meiboom-Gill) refocusing protocol. In certain cases, spectra were acquired using (1)H --> (14)N broadband cross-polarization, via the BRAIN-CP (broadband adiabatic inversion - cross polarization) pulse sequence. These spectra provide (14)N electric field gradient (EFG) tensor parameters and orientations that are particularly sensitive to variations in local structure and intermolecular hydrogen-bonding interactions. The (1)H{(15)N} idHETCOR spectra, acquired under conditions of fast magic-angle spinning (MAS), used CP transfers to provide (1)H-(15)N chemical shift correlations for all nitrogen environments, except for two sites in acebutolol and nicardipine. One of these two sites (RR'NH2(+) in acebutolol) was successfully detected using the DNP-enhanced (15)N{(1)H} CP/MAS measurement, and one (RNO2 in nicardipine) remained elusive due to the absence of nearby protons. This exploratory study suggests that this combination of techniques has great potential for the characterization of solid APIs and numerous other organic, biological, and inorganic systems.
Amphiphilic effects of dibucaine HCl on rotational mobility of n-(9-anthroyloxy)stearic acid in neuronal and model membranes.[Pubmed:17241620]
Chem Phys Lipids. 2007 Mar;146(1):33-42.
We studied dibucaine's effects on specific locations of n-(9-anthroyloxy)palmitic acid or stearic acid (n-AS) within phospholipids of synaptosomal plasma membrane vesicles isolated from bovine cerebral cortex (SPMV) and model membranes. Giant unilamellar vesicles (GUVs) were prepared with total lipids (SPMVTL) and mixture of several phospholipids (SPMVPL) extracted from SPMV. Dibucaine.HCl increased rotational mobility (increased disordering) of hydrocarbon interior, but it decreased mobility (increased ordering) of membrane interface, in both native and model membranes. The degree of rotational mobility in accordance with the carbon atom numbers of phospholipids comprising neuronal and model membranes was in the order at the 16, 12, 9, 6 and 2 position of aliphatic chain present in phospholipids. The sensitivity of increasing or decreasing effect of rotational mobility of hydrocarbon interior or surface region by dibucaine.HCl differed depending on the neuronal and model membranes in the descending order of SPMV, SPMVPL and SPMVTL.
False positives and false negatives with a cocaine-specific field test and modification of test protocol to reduce false decision.[Pubmed:16226152]
Forensic Sci Int. 2005 Dec 20;155(2-3):158-64.
The specificity of the Scott test, which is widely used in the field to detect cocaine, was investigated. Several drugs and medicines were applied to the test, and the conditions leading to false positives or false negatives were defined. The Scott test consists of three steps, each involving the addition of a certain reagent and observation of the color that consequently develops. In the first step, blue precipitates appear. In the second, these precipitates completely disappear. In the third step, blue appears again, but in the lower layer. It became clear that proper sample size is critical for correct decision, since too much heroin or dibucaine showed exactly the same color sequence as cocaine HCl and thus gave false positives, and too much cocaine HCl showed persisting precipitates in the second step, yielding a false negative. The appropriate sample size was 1mg or smaller. Freebase (crack) cocaine gave false negatives even when the sample size was appropriate, and it could not be distinguished from a newer substance of abuse, 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT, foxy). The authors developed a new protocol to distinguish crack from 5-MeO-DIPT.
[Glucose attenuating local anesthetic-induced hemolysis].[Pubmed:15500098]
Masui. 2004 Sep;53(9):994-7.
BACKGROUND: The effect of glucose on local anesthetic-induced neural damage has not been fully studied. We examined the effect of glucose on hemolysis induced by local anesthetics. METHODS: The mean EC50 values (the local anesthetic level that causes destruction of half of the red blood cells in vitro) of lidocaine HCl, tetracaine HCl and dibucaine HCl were determined with 0% and 7.5% glucose contained in Krebs solution at pH 6.4. RESULTS: The mean EC50 values of lidocaine HCl, tetracaine HCl, and dibucaine HCl in 0%-glucose Krebs solution were 6.51%, 0.45%, 0.17%, respectively, which increased significantly to 7.05%, 0.64% and 0.23%, in 7.5% glucose Krebs solution at pH 6.4. CONCLUSIONS: Glucose may have a protective role in local anesthetic-induced neural damage.
Preferential partitioning of uncharged local anesthetics into the surface-adsorbed film.[Pubmed:15465309]
Colloids Surf B Biointerfaces. 2004 Oct 10;38(1-2):91-9.
The surface tension and pH of aqueous solutions of three hydrochloric acid (HCl) - uncharged anesthetic (mepivacaine (MC), bupibacaine (BC) and dibucaine (DC)) mixtures were measured as a function of total molality and composition of local anesthetic in order to investigate the competitive surface-adsorption of uncharged and charged local anesthetics. The behavior of the surface tension versus total molality and pH versus total molality curves remarkably changed at the composition corresponding to an equimolar mixture. The pH measurements showed that uncharged and charged forms coexisted only at compositions more than the equimolar mixture. The partitioning quantities of respective uncharged and charged anesthetics into the surface-adsorbed film were estimated from their surface densities calculated thermodynamically. The greater quantity of uncharged anesthetics existed in the adsorbed film at the coexisting composition, that is, the uncharged anesthetics adsorbed more preferentially than charged ones. The relative ease with which uncharged anesthetics transferred into the surface-adsorbed film was proportional to the hydrophobicities and well correlated the anesthetic potencies. At compositions in the vicinity of physiological pH (ca. 7.4), the bulk solution is more abundant in charged anesthetics than uncharged ones, whereas the uncharged molecules is conversely more abundant in the surface region. The present results clearly imply that the surface-active molecule of local anesthetic in the physiological pH is the uncharged form and the partitioning is greatly dependent on the hydrophobicity among the anesthetics.


