CytidineCAS# 65-46-3 |
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Foscarnet Sodium
Catalog No.:BCC4782
CAS No.:63585-09-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 65-46-3 | SDF | Download SDF |
| PubChem ID | 596 | Appearance | Powder |
| Formula | C9H13N3O5 | M.Wt | 243.2 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (205.58 mM) DMSO : 50 mg/mL (205.58 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
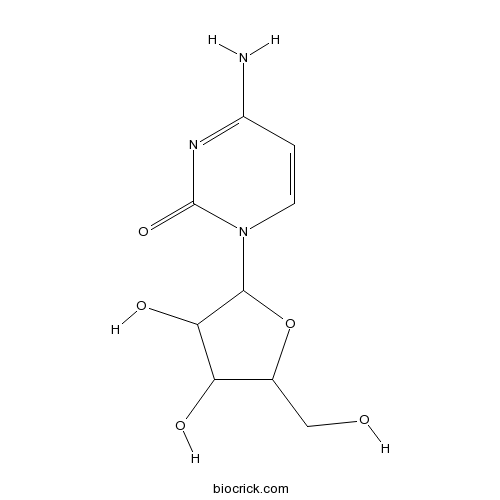
| Chemical Name | 4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one | ||
| SMILES | C1=CN(C(=O)N=C1N)C2C(C(C(O2)CO)O)O | ||
| Standard InChIKey | UHDGCWIWMRVCDJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring, cytidine is a component of RNA.Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. |
| Targets | HIV |
| In vitro | The effect of cytidine on the structure and function of an RNA ligase ribozyme.[Pubmed: 11333020]RNA. 2001 Mar; 7(3): 395–404.
|
| Kinase Assay | Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation[Reference: WebLink]Journal of Virology, 2007 , 81 (13) :7238.APOBEC3G (A3G) is a single-stranded DNA Cytidine deaminase that targets retroviral minus-strand DNA and has potent antiviral activity against diverse retroviruses. However, the mechanisms of A3G antiviral functions are incompletely understood. |

Cytidine Dilution Calculator

Cytidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1118 mL | 20.5592 mL | 41.1184 mL | 82.2368 mL | 102.7961 mL |
| 5 mM | 0.8224 mL | 4.1118 mL | 8.2237 mL | 16.4474 mL | 20.5592 mL |
| 10 mM | 0.4112 mL | 2.0559 mL | 4.1118 mL | 8.2237 mL | 10.2796 mL |
| 50 mM | 0.0822 mL | 0.4112 mL | 0.8224 mL | 1.6447 mL | 2.0559 mL |
| 100 mM | 0.0411 mL | 0.2056 mL | 0.4112 mL | 0.8224 mL | 1.028 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring, cytidine is a component of RNA.
- Nicotine Difartrate
Catalog No.:BCC3821
CAS No.:65-31-6
- Gallamine Triethiodide
Catalog No.:BCC4576
CAS No.:65-29-2
- Phentolamine Mesylate
Catalog No.:BCC4353
CAS No.:65-28-1
- Pyridoxine
Catalog No.:BCC8355
CAS No.:65-23-6
- Yohimbine Hydrochloride
Catalog No.:BCN6268
CAS No.:65-19-0
- 1-Testosterone
Catalog No.:BCC8474
CAS No.:65-06-5
- 7alpha-Hydroxystigmasterol
Catalog No.:BCN4194
CAS No.:64998-19-2
- Pifithrin-μ
Catalog No.:BCC2412
CAS No.:64984-31-2
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
- H-D-Asp(OtBu)-OH
Catalog No.:BCC2899
CAS No.:64960-75-4
- Ditryptophenaline
Catalog No.:BCN7408
CAS No.:64947-43-9
- 4-Aminosalicylic acid
Catalog No.:BCC8691
CAS No.:65-49-6
- Acridine Orange hydrochloride
Catalog No.:BCC8006
CAS No.:65-61-2
- Thymine
Catalog No.:BCN8334
CAS No.:65-71-4
- Ac-Met-OH
Catalog No.:BCC2991
CAS No.:65-82-7
- Benzoic acid
Catalog No.:BCN4201
CAS No.:65-85-0
- Orotic acid
Catalog No.:BCC4162
CAS No.:65-86-1
- 1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No.:BCN7347
CAS No.:65008-02-8
- 3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No.:BCN1387
CAS No.:65008-17-5
- VGX-1027
Catalog No.:BCC5203
CAS No.:6501-72-0
- Boc-His(Tos)-OH.DCHA
Catalog No.:BCC2605
CAS No.:65057-34-3
- Nimorazole
Catalog No.:BCC5253
CAS No.:6506-37-2
- 6-Amino-2-methylquinoline
Catalog No.:BCC8759
CAS No.:65079-19-8
The effect of cytidine on the structure and function of an RNA ligase ribozyme.[Pubmed:11333020]
RNA. 2001 Mar;7(3):395-404.
A Cytidine-free ribozyme with RNA ligase activity was obtained by in vitro evolution, starting from a pool of random-sequence RNAs that contained only guanosine, adenosine, and uridine. This ribozyme contains 74 nt and catalyzes formation of a 3',5'-phosphodiester linkage with a catalytic rate of 0.016 min(-1). The RNA adopts a simple secondary structure based on a three-way junction motif, with ligation occurring at the end of a stem region located several nucleotides away from the junction. Cytidine was introduced to the Cytidine-free ribozyme in a combinatorial fashion and additional rounds of in vitro evolution were carried out to allow the molecule to adapt to this added component. The resulting Cytidine-containing ribozyme formed a 3',5' linkage with a catalytic rate of 0.32 min(-1). The improved rate of the Cytidine-containing ribozyme was the result of 12 mutations, including seven added Cytidines, that remodeled the internal bulge loops located adjacent to the three-way junction and stabilized the peripheral stem regions.


