CamptothecinTopoisomerase I inhibitor,prototypic CAS# 7689-03-4 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
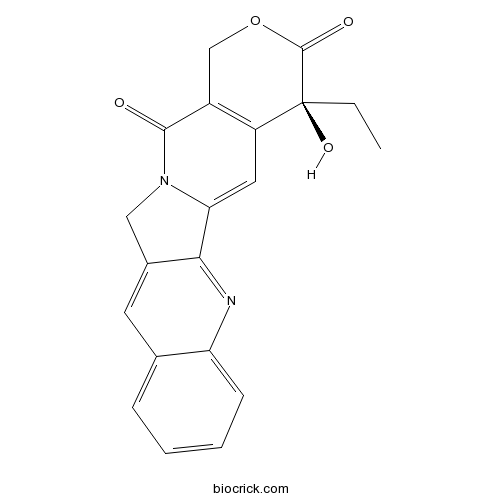
| Cas No. | 7689-03-4 | SDF | Download SDF |
| PubChem ID | 24360 | Appearance | Yellow powder |
| Formula | C20H16N2O4 | M.Wt | 348.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Camptothecin; (S)-(+)-Camptothecin; CPT | ||
| Solubility | DMSO : 7.69 mg/mL (22.08 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CCC1(C2=C(COC1=O)C(=O)N3CC4=CC5=CC=CC=C5N=C4C3=C2)O | ||
| Standard InChIKey | VSJKWCGYPAHWDS-FQEVSTJZSA-N | ||
| Standard InChI | InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Camptothecin is a specific inhibitor of DNA topoisomerase I (Topo I) with IC50 of 0.68 μM; it is a novel alkaloidal leukemia and tumor inhibitor, is a strong inhibitor of nucleic acid synthesis in mammalian cells and a potent inducer of strand breaks in chromosomal DNA. Camptothecin and its analogs reduce amyloid-β production and amyloid-β42-induced IL-1β production. |
| Targets | Beta Amyloid | p53 | IL Receptor | Topoisomerase |
| In vitro | Camptothecin and its analogs reduce amyloid-β production and amyloid-β42-induced IL-1β production.[Pubmed: 25096614]J Alzheimers Dis. 2015;43(2):465-77.Compounds derived from natural products are becoming promising alternative drugs/tools in Alzheimer's disease (AD) therapeutics.
|
| In vivo | Development and evaluation of magnetic microemulsion: tool for targeted delivery of camptothecin to BALB/c mice-bearing breast cancer.[Pubmed: 25119147]J Drug Target. 2014 Dec;22(10):913-26.Development and evaluation of Camptothecin-loaded-microemulsion (ME) and -magnetic microemulsion (MME) for passive/active-targeted delivery to BALB/c mice-bearing breast cancer.
|
| Kinase Assay | Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I.[Pubmed: 2997227]Pharmacodynamic and pharmacogenomic study of the nanoparticle conjugate of camptothecin CRLX101 for the treatment of cancer.[Pubmed: 24768630]Nanomedicine. 2014 Oct;10(7):1477-86.CRLX101 is a nanopharmaceutical consisting of cyclodextrin-based polymer molecule and Camptothecin. The CRLX101 nanoparticle is designed to concentrate and slowly release Camptothecin in tumors over an extended period of time.
J Biol Chem. 1985 Nov 25;260(27):14873-8.Camptothecin, a cytotoxic drug, is a strong inhibitor of nucleic acid synthesis in mammalian cells and a potent inducer of strand breaks in chromosomal DNA. |

Camptothecin Dilution Calculator

Camptothecin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8703 mL | 14.3513 mL | 28.7026 mL | 57.4053 mL | 71.7566 mL |
| 5 mM | 0.5741 mL | 2.8703 mL | 5.7405 mL | 11.4811 mL | 14.3513 mL |
| 10 mM | 0.287 mL | 1.4351 mL | 2.8703 mL | 5.7405 mL | 7.1757 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.5741 mL | 1.1481 mL | 1.4351 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Camptothecin is a selective inhibitor of topoisomerase I with IC50 value of 679 nM [1].
Camptothecin could induce cell death in SMMC-7721, MCF-7, and HCT-116 tumor cells. Camptothecin has been reported to induce autophagy via AMPK-TSC2-mTOR pathway, at the mean time, induce premature senescence by ATM-Chk2-p53-p21 pathway [2].
Studies indicated that camptothecin had anti-tumor activity in (non-small cell lung cancer) NSCLC xenografts. Camptothecin -induced DNA damage has revealed to cause phosphorylation of H2AX (γH2AX). Previous studies have also demonstrated to reduce the expression of Top1 in cancer cells treated with camptothecins [3].
References:
[1] Luzzio MJ1, Besterman JM, Emerson DL, Evans MG, Lackey K, Leitner PL, McIntyre G, Morton B, Myers PL, Peel M, et al. Synthesis and antitumor activity of novel water soluble derivatives of camptothecin as specific inhibitors of topoisomerase I. J Med Chem. 1995 Feb 3;38(3):395-401.
[2] Zhang JW1, Zhang SS1, Song JR2, Sun K3, Zong C1, Zhao QD1, Liu WT1, Li R1, Wu MC1, Wei LX4. Autophagy inhibition switches low-dose camptothecin-induced premature senescence to apoptosis in human colorectal cancer cells. Biochem Pharmacol. 2014 May 22. pii: S0006-2952(14)00286-X.
[3] Tsakalozou E1, Adane ED, Liang Y, Arnold SM, Leggas M. Protracted dosing of the lipophilic camptothecin analogue AR-67 in non-small cell lung cancer xenografts and humans. Cancer Chemother Pharmacol. 2014 May 8. [Epub ahead of print]
- Olivil 4'-O-glucoside
Catalog No.:BCN7557
CAS No.:76880-93-8
- 8-Methyl-8-azabicyclo[3.2.1]octane-3,6-diol, 9CI; (3RS,6RS)-form, 3-O-Ac
Catalog No.:BCN1361
CAS No.:7688-76-8
- 6-Hydroxywogonin
Catalog No.:BCN6556
CAS No.:76844-70-7
- Przewaquinone A
Catalog No.:BCN3004
CAS No.:76843-23-7
- 5-BDBD
Catalog No.:BCC7717
CAS No.:768404-03-1
- Famotidine
Catalog No.:BCC4529
CAS No.:76824-35-6
- Danshensu
Catalog No.:BCN8513
CAS No.:76822-21-4
- Pimaricin
Catalog No.:BCN2216
CAS No.:7681-93-8
- Ronidazole
Catalog No.:BCC4840
CAS No.:7681-76-7
- Potassium Iodide
Catalog No.:BCC4826
CAS No.:7681-11-0
- Azathramycin
Catalog No.:BCC1392
CAS No.:76801-85-9
- 3-Hydroxy-4',5,7-trimethoxyflavanone
Catalog No.:BCN4316
CAS No.:76792-94-4
- Bz-Gly-OH.HCl
Catalog No.:BCC2945
CAS No.:7689-50-1
- Triacsin C
Catalog No.:BCC7377
CAS No.:76896-80-5
- 13-Methyl-8,11,13-podocarpatriene-3,12-diol
Catalog No.:BCN1360
CAS No.:769140-74-1
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
- Viscosalactone B
Catalog No.:BCN7945
CAS No.:76938-46-0
- 8-Bromo-cAMP, sodium salt
Catalog No.:BCC8078
CAS No.:76939-46-3
- Onitin 2'-O-glucoside
Catalog No.:BCN4319
CAS No.:76947-60-9
- Cleomiscosin A
Catalog No.:BCN4320
CAS No.:76948-72-6
- DL-alpha-Tocopherylacetate
Catalog No.:BCN2904
CAS No.:7695-91-2
- Kalii Dehydrographolidi Succinas
Catalog No.:BCN8523
CAS No.:76958-99-1
- Nizatidine
Catalog No.:BCC4522
CAS No.:76963-41-2
- H-D-2-Nal-OH.HCl
Catalog No.:BCC3286
CAS No.:76985-09-6
Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I.[Pubmed:2997227]
J Biol Chem. 1985 Nov 25;260(27):14873-8.
Camptothecin, a cytotoxic drug, is a strong inhibitor of nucleic acid synthesis in mammalian cells and a potent inducer of strand breaks in chromosomal DNA. Neither the equilibrium dialysis nor the unwinding measurement indicates any interaction between Camptothecin and purified DNA. However, Camptothecin induces extensive single strand DNA breaks in reactions containing purified mammalian DNA topoisomerase I. DNA breakage in vitro is immediate and reversible. Analyses of Camptothecin-induced DNA breaks show that topoisomerase I is covalently linked to the 3' end of the broken DNA. In addition, Camptothecin inhibits the catalytic activity of mammalian DNA topoisomerase I. We propose that Camptothecin blocks the rejoining step of the breakage-reunion reaction of mammalian DNA topoisomerase I. This blockage results in the accumulation of a cleavable complex which resembles the transient intermediate proposed for eukaryotic DNA topoisomerase I. The inhibition of nucleic acid synthesis and the induction of DNA strand breaks observed in vivo may be related to the formation of this drug-induced cleavable complex.
Topoisomerase I inhibitors: camptothecins and beyond.[Pubmed:16990856]
Nat Rev Cancer. 2006 Oct;6(10):789-802.
Nuclear DNA topoisomerase I (TOP1) is an essential human enzyme. It is the only known target of the alkaloid Camptothecin, from which the potent anticancer agents irinotecan and topotecan are derived. As Camptothecins bind at the interface of the TOP1-DNA complex, they represent a paradigm for interfacial inhibitors that reversibly trap macromolecular complexes. Several Camptothecin and non-Camptothecin derivatives are being developed to further increase anti-tumour activity and reduce side effects. The mechanisms and molecular determinants of tumour response to TOP1 inhibitors are reviewed, and rational combinations of TOP1 inhibitors with other drugs are considered based on current knowledge of repair and checkpoint pathways that are associated with TOP1-mediated DNA damage.
Development and evaluation of magnetic microemulsion: tool for targeted delivery of camptothecin to BALB/c mice-bearing breast cancer.[Pubmed:25119147]
J Drug Target. 2014 Dec;22(10):913-26.
PURPOSE: Development and evaluation of Camptothecin-loaded-microemulsion (ME) and -magnetic microemulsion (MME) for passive/active-targeted delivery to BALB/c mice-bearing breast cancer. METHODS: Based on the pseudo-ternary phase diagrams Camptothecin-loaded-MEs and -MMEs were developed using benzyl alcohol:Captex 300 (3:1), TPGS:Tween 80 (2:1) and water. Furthermore, characterized for their droplet size distribution, magnetic susceptibility and effect of droplet size in plasma and evaluated for in vitro and in vivo targeting potential, drug release, haemolytic potential, cytotoxicity, genotoxicity, in vivo biodistribution and lactone ring stability. RESULTS: Drug-loaded MEs showed uniform droplet distribution, extended drug release (76.07 +/- 4.30% at 24 h), acceptable level of haemolytic activity (<20%), significant cytotoxicity (129 +/- 3.9 ng/mL) against MCF-7 cancer cells and low DNA damage in lymphocytes. Targeting potential of MMEs was documented in 4T1 breast cancer-induced BALB/c mice. MMEs were concentrated more at the target tissue on introduction of external magnetic field. In vivo biodistribution study documented the active targeting of 5067.56 +/- 354.72 ng/gm and passive targeting of 1677.58 +/- 134.20 ng/gm Camptothecin to breast cancer from MME and ME, respectively. Lactone stability study shows around 80% of the lactone stable at 24 h. CONCLUSIONS: Developed ME and MME may act as a promising nanocarrier for efficient targeting of breast cancer tissues.
Camptothecin and its analogs reduce amyloid-beta production and amyloid-beta42-induced IL-1beta production.[Pubmed:25096614]
J Alzheimers Dis. 2015;43(2):465-77.
Compounds derived from natural products are becoming promising alternative drugs/tools in Alzheimer's disease (AD) therapeutics. From an in-house natural products library, seventeen hits were selected for their inhibitory effect on the production of amyloid-beta (Abeta) with IC50 lower than 10 muM without causing obvious toxicity. Among these compounds, Camptothecin (CPT) and its analogs showed inhibitory effects on amyloid-beta 1-42 (Abeta42) with the IC50 value in the nanomolar range in HEKsw cells and SHSY5Ysw cells. Further studies showed that CPT and its analogs inhibited Abeta42 via a p53 dependent pathway. Meanwhile, CPT and its analogs could also inhibit Abeta42 induced IL-1beta production in the THP-1 cells. Taken together, our results indicate that CPT and its analogs would be a promising therapeutic candidates for AD.
Pharmacodynamic and pharmacogenomic study of the nanoparticle conjugate of camptothecin CRLX101 for the treatment of cancer.[Pubmed:24768630]
Nanomedicine. 2014 Oct;10(7):1477-86.
CRLX101 is a nanopharmaceutical consisting of cyclodextrin-based polymer molecule and Camptothecin. The CRLX101 nanoparticle is designed to concentrate and slowly release Camptothecin in tumors over an extended period of time. Tumor biopsy and blood samples collected from patients with advanced solid malignancies before and after CRLX101 treatment are subjected to immunohistochemistry and pharmacogenomics. The expression of Topoisomerase-1, Ki-67, CaIX, CD31 and VEGF decreased after CRLX101 treatment. The expressions of these proteins are inversely proportional with survival duration of the patients. The Drug Metabolism Enzymes and Transporters (DMET) array shows an allele frequency in patients similar to global populations with none of the SNPs associated with toxicity. The results suggest that the observed lower toxicity is not likely to be due to different genotypes in SNPs. CRLX101 demonstrates a promising anti-tumor activity in heavily pre-treated or treatment-refractory solid tumor malignancies presumably by inhibition of proliferation and angiogenesis correlating with tumor growth inhibition. From the clinical editor: In this cancer treatment study clinical samples collected from patients were subjected to immunohistochemistry and pharmacogenomics. The expressions of key proteins that are inversely proportional with survival duration of the patients decreased after treatment with CRLX101, a Camptothecin slow-release nanoparticle conjugate. This anti-tumor activity in heavily pre-treated and treatment resistant solid tumors, promises a novel therapeutic approach.
Camptothecin derivatives induce regression of human ovarian carcinomas grown in nude mice and distinguish between non-tumorigenic and tumorigenic cells in vitro.[Pubmed:8449612]
Int J Cancer. 1993 Mar 12;53(5):863-71.
We have recently shown that the plant alkaloid 20(S)-Camptothecin and its derivatives 9-nitro-20(S)-Camptothecin(9NC) and 9-amino-20(S)-Camptothecin(9AC) inhibit the growth of a variety of human tumors xenografted in nude mice. In this report, we demonstrate that 9NC and 9AC effectively inhibit growth, and subsequently induce regression, of human ovarian tumors grown in nude mice. Tumor regression is accompanied by degenerative changes in the tumor cells as assessed by microscopic observations of histological sections prepared from the tumors. Parallel experiments in vitro show that 9NC inhibits in a similar manner the growth of human ovarian carcinoma cells, regardless of their ability to induce tumors when xenografted in nude mice, and induces similar morphological changes in both non-tumorigenic and tumorigenic cells, as assessed by microscopic observation. Flow cytometry studies show that 9NC-induced growth inhibition of the non-tumorigenic cells is associated with accumulation of these cells in G2. In contrast, 9NC-induced growth inhibition of the tumorigenic cells is associated with the generation of cells containing a reduced DNA content, that is, cells programmed to die. In conclusion, Camptothecins appear to be cytostatic for non-tumorigenic, but cytotoxic for tumorigenic cells, an important finding from viewpoints of cell biology, pharmacology and cancer chemotherapy.


